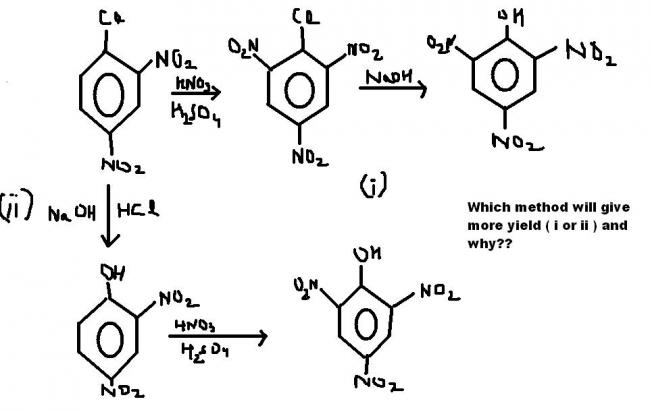
i also think its the second path bcoz of less deactivated ring.
22 Answers
and rohit 11 halogens are weakly deactivating groups...not weakly activating
but Rohit in the book it is given that second method will give more yield!!
this reaction will occur by itself...as the NO2 groups are there...higher temperature will b needed for the second method....but for the first method normal temperature will do the reaction...:)
yeah, organic is saying right too, OH is activating the benzene ring while halide group is a weakly activating group, so an electrophile will prefer OH as comapre to Cl
I think it should be method 2 because: Cl shows -I effect , while OH shows -I and +R effects, upcoming group in II is a nitro group(electrophile), so it willl prefer to accept +R , -I effect of OH other than -I effect of Cl in I . Please comment
what is the answer given in the book from which the question is taken?
i spot a problem here.....how are simply using NaOH and HCl to convert a aromatic halide group to a phenolic OH group??
The second method gives more yield because the NO2 +(electrophile) prefers to attack the more electron rich species at the more electron rich position..the OH gropu makes the ortho position more electron rich than the Cl group !
i think method 1 has more yield.... because of excessive steric hinderence Cl comes outta the plane of the benzene ring and can easily be replaced by OH
plzz.....guys koi to madad karo.....sab reaction mein galtiyaan nikal kar chale gaye!! hehe....!!
ok make it NaOH + HCl at 368 K thats all . You dont need any extreme conditions because of the two NO2 groups attached to the ring.........if it would have been simply clorobenzene then needed extreme conditions like 625 k temp and 300 atm....hope it helps u
aryl halide cannot be directly converted to phenol.extreme conditions are must.read ncert dude
i mean i dont fin a mistake in this.......see any book and aryl halide is converted to phenol in presence of NaOH and H+ only
the main flaw that appeals to me is that the methods have used NaOH (without any extremes of conditions) to convert an aromatic halide group to a phenolic OH group !
yeah i agree with organic.There can be neutralization rxn between the regents,also the rxn shud be carried only on a basic medium.
what are u saying mr psycho....how should i correct the conversion? there are multiple ways to do so!!! i am expecting a reply from the originator of this thread! hope he responds soon!
ok...organic,,,den wat is d flaw in d conversion....u make corrections to it and post it here...