6 Answers
abhishek sahoo
·2010-03-22 08:45:03
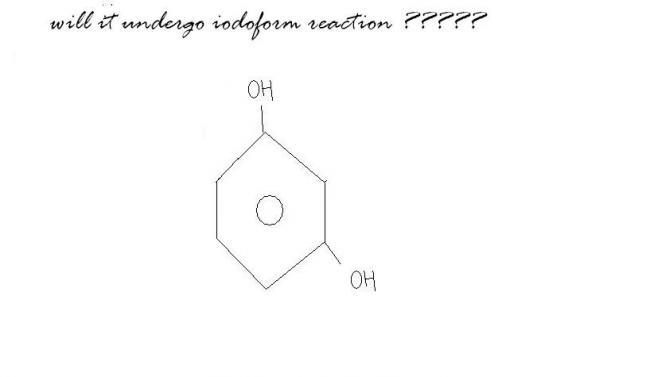
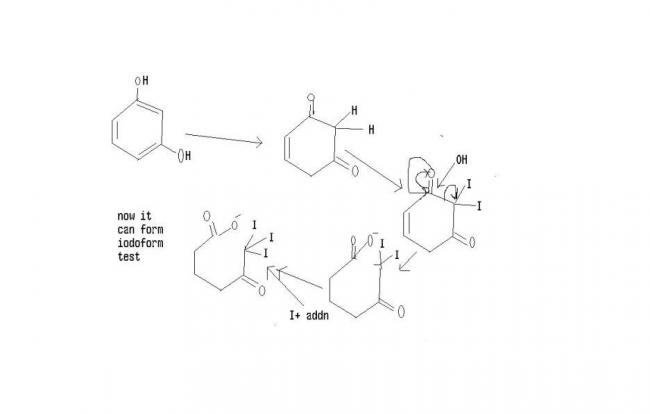
yes..... in 1.3 dihydroxy when it undergoes tautomerisation it forms a diketone whish is unstable soon react with base to undergoes furthur idoform reaction '' CH2 is acidic in nature"
Pritish Chakraborty
·2010-03-22 09:09:37
In my notes it's given that this does undergo haloform..strange.
Tush Watts
·2010-03-22 10:07:32
Yes it will
Halogen substituted keto methyl group can also respond to haloform reacn