okkkkkkkk..
really nice thing....i never knew abt that ...
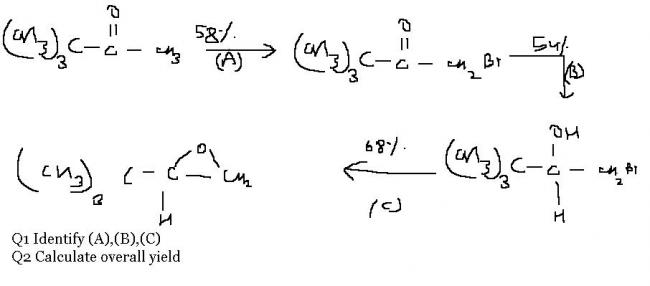
my doubt is not Q1 but Q2
how to find overall yield????
-
UP 0 DOWN 0 0 2

2 Answers
Asish Mahapatra
·2009-09-29 21:45:40
suppose you take 100 moles of the reactant.
Then according to data only 58 moles of the next intermediate product will form.
After that 54% of this 58 moles will form . i.e. 31.32 moles
next there is 68% yield. So final no. of moles formed = 68% of 31.32 moles = 21.2976
So final percentage yield = 21.2976 %
BTW, what are (A) (B) (C)