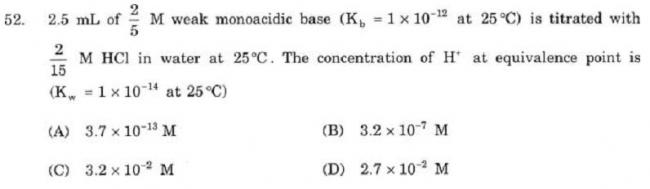
1
1let V be the volume of HCl used...
2.5 * 2/5 = V * 2/15
V= 7.5 ml.....
total volume = 10 ml...
Conc. of salt produced (c) = (2.5 * 2/5)/10
= 0.1 M
Kh= Kw/Kb
= 10-14/10-12
=10-2
for hydrolysis of base..
B+ +H2O -> BOH + H+
c 0 0
c(1-h) ch ch
Kh= ch2/(1-h)
Putting the values of Kh = 10-2 and c= 0.1 M
We get h= 0.27
[H+] = 0.1 * 0.27 = 2.7 * 10-2 M
 1
1that will be when in equation ch2/1-h u take 1-h ~ 1
 1
1i have done it by that too....
 1
1bhaiya what is the correct answer.....
 1
1bhaiya iska answer toh do.....
 11
11Ans D
http://www.iitkgp.ernet.in/jee/paper12008.pdf