well, even i got a !
The correct acidity order of the following is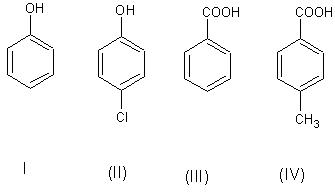
(A)III>IV>II>I
(B)IV>III>I>II
(C)III>II>I>IV
(D)II>III>IV>I
-
UP 0 DOWN 0 0 12

12 Answers
[url=http://iitjee2009solutions.targetiit.com]Solution Key with Question Paper of 2009 IIT JEE[/url]
well bhaiyaa i think that its C because the anion formed after deprotonation is most stable in C!
IV should be the least acidic and most basic as it shows +I to it!!!!!
cheers!!!!!!!!
but CH3 willl show +I effect and hence wil reduce acidity whereas Cl and OH will show -I effect and will increase acidity therefore answer must be (c)
plzz explain
@ jesh ... see the 4th compound has an cooh group .. its an acid ..it is going to be more acidic than chloro phenol
We decide by inductive effect here...using resonance is pretty much futile as OH is anyway +R and COOH is -R...doesn't help our cause. Had there been substrates where in one resonance is present and not in the other comparison would've worked that way.
Using inductive effect is much quicker. Benzoic acids are obviously more acidic than phenols. Let's decide phenols first. Chlorine exerts a huge -I effect which engulfs its tiny +R effect, so like I said we use inductive here. So chlorophenol is more acidic than phenol.
In the benzoic acids, methyl group provides +I effect which diminishes the acidity of the acid to some extent. So II > I and IV < III
The overall order becomes III > IV > II > I which is (A).
ans is a
benzoic acid wheather substituted or nt is always strong acid thn phenol
d has pKa arnd 4
b has pKa arnd 7 or 8
so d is more acidic