2.> exothermic reaction occurs>>increasing temp favours backward reaction...(b)
3.>SN2 means the rate follows bimolecular path ie.both halide as well as OH- will depend..now u can calc to get the answer
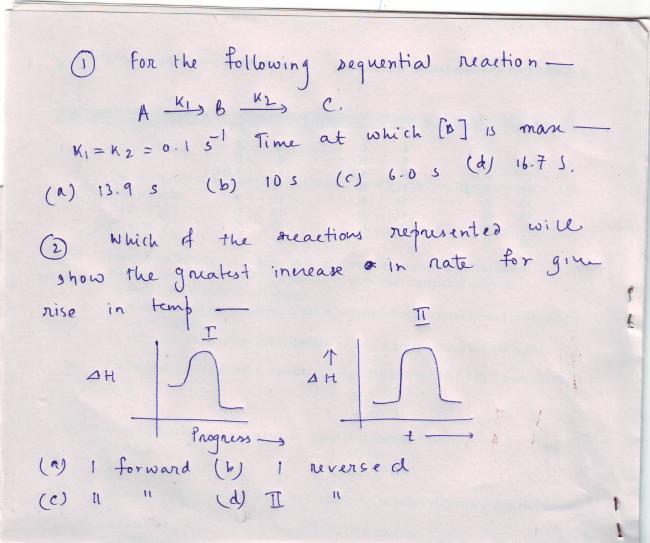
for q1..im not sure is the ans:10 seconds
 please explain the concept clearly
please explain the concept clearly
Q3.for a reaction ,rate expressions is given as----- RX+OH-=ROH +X- rate=4.7*10-5[RX][OH]+0.24*10-5[RX] wHAT PERCENT OF RX REACT BY SN2 ?WHEN [OH]=0.001M.
2.> exothermic reaction occurs>>increasing temp favours backward reaction...(b)
3.>SN2 means the rate follows bimolecular path ie.both halide as well as OH- will depend..now u can calc to get the answer
for q1..im not sure is the ans:10 seconds
for question 3--
for SN2 rekn.
for first case % of this reaction takes place by the SN2 mechanism =
4.7*10-5[RX][OH]4.7*10-5[RX][OH]+0.24*10-5[RX]
now put the value of [OH]=0.001M. and cancel [RX] and get the answer
1 requires solving the differential equations
dA/dt=-k1A
dB/dt = k1A-k2B
dC/dt = k2B
if B is max then dB/dt =0
A=A0e-k1t
Now solve...