8. How (H +1 and O -2 ??) but what abt P in PH3
6. How
5. kaun se chapter mein hai?
Q1. Which contains both polar and non-polar bonds?
(a) NH4Cl (b) HCN (c) H2O2 (d) CH4
Q2. Spin magnetic moment in BM of Hg[Co(SCN)4] is
(do we consider S as donor atom or N?)
Q3. Ionic radii of
(a) Ti4+<Mn7+ (b) 35Cl- < 37Cl- (c) K+>Cl- (d) P3+>P5+
how to compare (b)??
Q4. Oxidation state of Cr and IUPAC name of
K2[Cr(CN)4NH3(NO)]
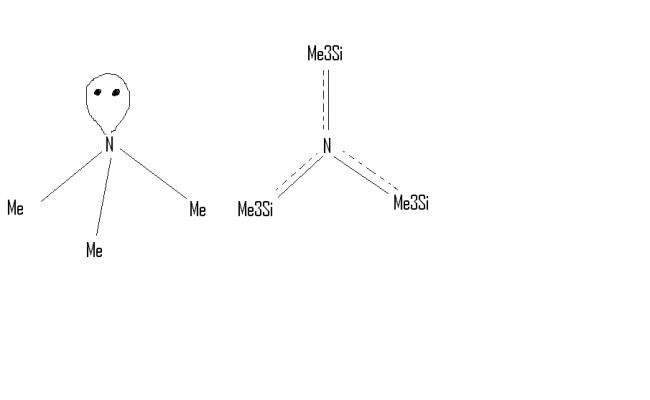
Q5. Are N(Me3) and N(SiMe3)3 isostructural?
Q6. In OSF4, O is present in axial/equatorial plane??
Q7. Order of H-bonding in S and Cl
Q8. Oxidation state of P in PH3 and N in NH2OH
-
UP 0 DOWN 0 2 10

10 Answers
1. (c) H2O2 HAS THE STRUCTURE - H------O----O-----H
-----O------O----- BOND IS NONPOLAR WHEREAS O----H BOND IS POLAR.
3. (d) more +ve charge means less radius.
4. IUPAC name of compound is Potassiumamminetetracyanonitroso chromate(I)
oxidation state of Cr is I
5. not isostructural. N(Me)3 is trigonal pyramidal and N(SiMe3)3 is trigonal planar because Silicon uses vacant d-orbital for backbonding with lone pairs of N-atom.
6. O is in equatorial posn.
7. S<Cl
Sorry but can't tell about 2. and 8.
1. thx but answer was given as NH4Cl. .. although i go with H2O2
3. I wanted how do u make sure abt (b) .. (d) is obviously correct
4,5 thx
6. why?
7. ans given S>Cl
Ans 1 C H2O2 contain a non-polar bond b/w O-O and polar bonds b/w O-H...
Ans 2 Consider S as a donor in SCN- and N as a donor in NCS-
Ans 3 There wont be a difference b/w the ionic radii in B coz neutrons reside in the nucleus and the nucleus is deep buried inside the atom and has radius of the order 10-15 m whereas the radius of atom is generally of the order 10-10 ..so number of neutrons inside the nucleus have no effect on size of the atom...
Ans 5 ..no they are not isostructural..coz the lone of pair of nitrogen is delocalised in the d orbitals of silicon so N(SiMe3)3 attains a planar structure whereas N(Me)3 is pyramidal..
Ans 6 It is present in equatorial..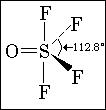
Ans 8 oxidation state of N in NH2OH is -1
Ans 5 ...chemical bondind...it is an exception...
Ans 8 draw the structure of NH2OH u will get the answer...N binded to 2 H and one oxygen which is further binded to H..
Oxidation state of P in PH3 is +3

Source: NCERT class 11th part 2 ..chapter Hydrogen...
5. 
6. 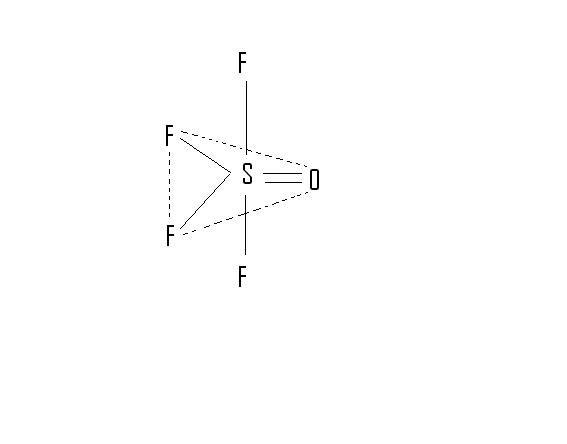 From the structure i think the position in which O is there is called equatorial
From the structure i think the position in which O is there is called equatorial
@ govind:
If in PH3 ..ON of P is +3 then how is this reaction disproportionation?
P4 + NaOH + H2O --> NaH2PO2 + PH3
Asish ...ur reason is true...but NCERT i dont think will be wrong...and wat abt the electronegativity values..Hydrogen 2.2 and phosphorus 2.1...experts plz help...
Tht reaction is Disproportionation for sure.
But, as "P" & "H" are nearly of the same electro-vities,,,,the oxidation state thing seems confusing.
Btw see these-
* http://docs.google.com/viewer?a=v&q=cache:apX-TtIIbJ8J:www.roanestate.edu/faculty/condon/blackbook/CHEM1110/oxidnumbans.pdf+PH3+oxidations+number&hl=en&gl=in&pid=bl&srcid=ADGEESg2bsf0OgIKVa2sSJTnFjiQ8mo2p_1ZMf0DgxNeqZf3joocKaBax-rYsXGMlZN92zGtHEriLyp50E6wLDGcAV6lujLEi2ezAmTxatIufUe4jL9mDVYs5GI7s163m_dnBemfRUV_&sig=AHIEtbSXxPYkWW4bptHcsCLfAdcpFw9F5g
----(after rule 4, they have taken -3 even fr As in AsH3!)
* http://answers.yahoo.com/question/index?qid=20080426102947AA1HMsL