Three 1L bulbs shown.Bulb A contains a mixture of H2O,CO2,N2 at 25C (All in gaseous state) and total presure 564 mm of Hg.Bulb B i sempty and at temp -70 C.Bulb C is also empty and at temp of -190 C.
CO2 sublimes at -78 C and N2 bolis at -196 C
Q1 If stop cock b/w A and B opened and system examined after loong time.Pressure in A and B is 219 mm Hg.What do bulb A and B contain ?
Q2 If both stop cocks opened,and system allowed to come into eq.Fnal pressure ios noted ass 33.5 mm of Hg.WHat do bulbs A,B,C contain ?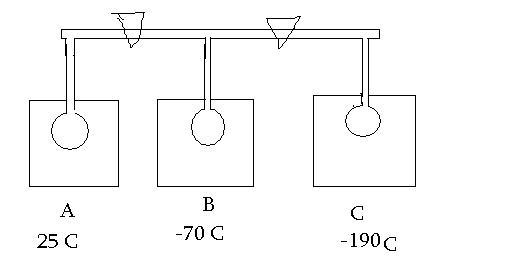
-
UP 0 DOWN 0 0 3

3 Answers
Case 1:
bulbs are maintained at the temp mentioned whether the valves are open or not.
SInce bulb B is at (-) 70 C, water vapour will condense to liquid and then to ice. This will reduce the water vpaour content (or partial pressure in bulb B and water vapour will transfer from A to B.
At equilibrium, the total pressures as well as the individual partial pressures in all the bulbs are equal. If the partial pressures are not equal, diffusion (mass transfer) takes place....
So as water vapour transfers from A to B, it keeps on condensing and freezing...
A will contain CO2 N2,H20g;(as water may not hav condensed completely)
B will contain CO2, N2 and ice.
based on tha data provided. Initial pressure Po, volume of all the bulbs V, Ta, Tb, Tc respective temp.
PoV = N R Ta; N initial total moles, Na, Nb, Nc moles in respective bulbs
case 1: P1 V = Na R Ta; P1V = Nb R Tb; N= Na + Nb
rearrangement yields: Nb/ Na = Ta/ Tb; Na = N/ (1+ Ta/Tb); P1= Po (Na/N)= Po /(1+Ta/Tb)
since Ta= 298, Tb= 203... P1 should have been 228, which is given as 219, indicating loss of moles from gaseous state...
Case2: A will contain only N2, B will contain N2, water ice, C (may contain water ice depending upon which valve was opened first and for how long), dry ice, and N2.
Calculations will show that in absence of condensation, the pressure in A should have been Po/ (1+Ta/Tb + Ta/Tc) or 93 mm Hg...
ans1
A=N2(g),CO2(g),H2O(g)
B=N2(g),CO2(g),H2O(s)
ans2
A=N2(g)
B=N2(g),H2O(s)
C=N2(g),CO2(s)