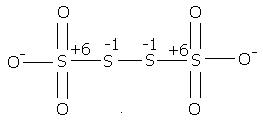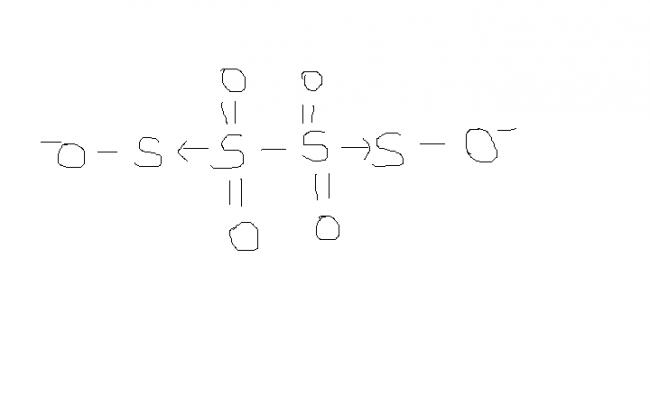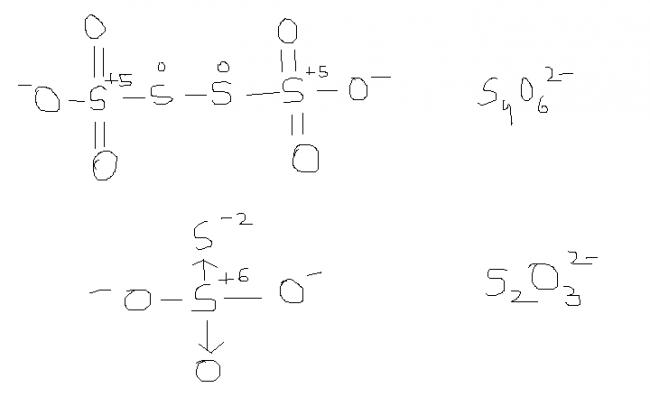
1) A solution of 0.2 gm of a compound containing Cu2+ and C2O42- ions on titration with 0.02 M KMnO4 in the presence of H2SO4 consumes 22.6 ml of the oxidant. The resulting solution is neutralized with Na2CO3, acidified with dil. acetic acid and treated with excess KI. The liberated iodine requires 11.3 ml 0.05 M Na2S2O3 for complete reduction, find out the mole ratio of Cu2+ to C2O42- in the compound
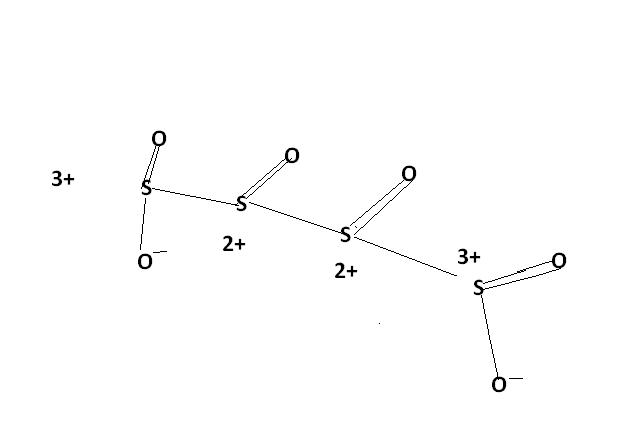
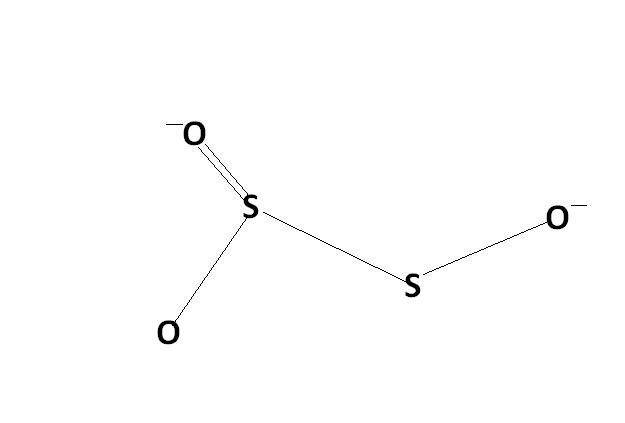
2) find the oxidation number of each sulphur atom in Na2S2O3 and Na2S4O6
3) Balance the following using oxidation number method:
FeS2+O2→Fe2O3+SO2
4) The electronic configuration of an element is 1s22s2......3p63d5. This represents its
(a) excited state (b) ground state (c) cationic form (d) anionic form
5)The velocity of electron in a certain Bohr’s orbit of hydrogen atom bears the ratio of 1 : 275 to the velocity of light
a) What is the quantum number (n) of orbit ?
b) Calculate the wave number of radiations emitted when electron jumps from (n+1) state to ground state
6) A 40 ml solution of weak base BOH is titrated with 0.1 N HCl solution. The pH of the solution is found to be 10.04 and 9.14 after adding 5 ml and 20 ml of the acid respectively. Find the dissociation constant of base
7) A sample of AgCl was treated with 5 ml of 1.5 M Na2CO3 solution to give Ag2CO3. The remaining solution contained 0.0026 g of Cl- per litre. Calculate the solubility product of AgCl (Ksp(Ag2CO3)=8.2×10-12)
-
UP 0 DOWN 0 1 26

26 Answers
Sir , i had read about this structure and it ws also specified dat " while considering redox reactions of
this type of compound , having variable oxidation states of a single atom , the AVERAGE oxidation state is considered. Here , the avg. oxidation state here of S is 2.5.and thus , S atom is oxidised frm 2 to 2.5 .
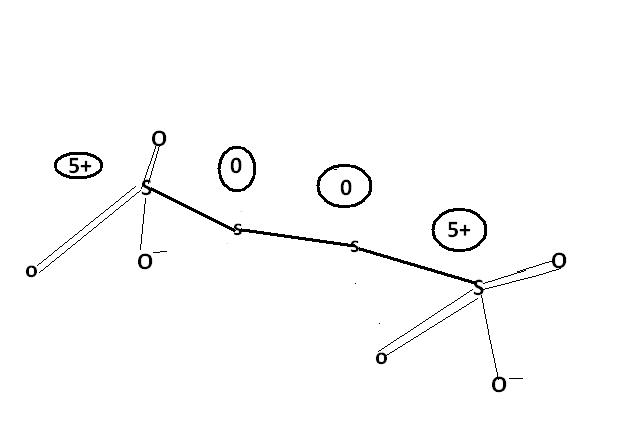
u cn also consider this structure.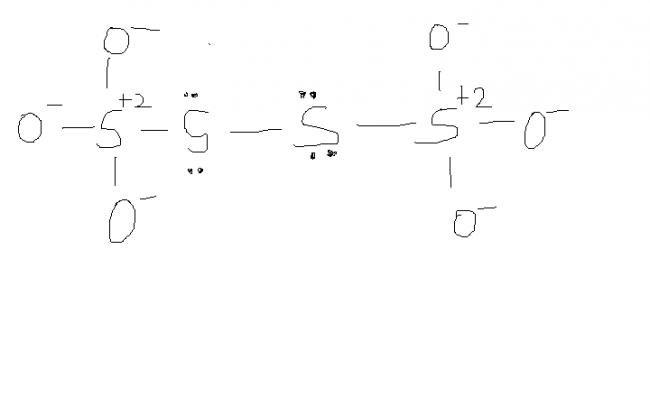
might be. actually , i'm hving d concept dat S-S bond will not carry any charge unless its S→S or S=S or S-S-Mn- / n+. please rectify me if i'm wrong.
5) formulas such as : VELOCITY ( V) =( Z2 / n2 ) * 2.18 * 106 m/s
and wave number (v) = Z2 RH ( 1/n12 - 1/n22 ) , where RH = 1.097 * 107 m-1 ( rydberg constant )
cn be used.
@ manish sir , r u sure , this structure is d same one. as here extreme S are at -1 oxidation state by BOND WITH O- and the middle S's are at +6 state by the presence of coordination bond.
I was just saying that in S4O62- there is a possibility of ON as +6, -1, -1, +6
which I think is more accurate.
What do you think?
s2o3 is thio -sulphate ox. stae of S is +3 & +3
s4o6 is also thio -sulphate ox.state is +3,+2,+2,+3
For Na2S2O3 it is correct,
but see again for S4O62- I have a doubt
Because with iodine it acts as reducing agent. and sulphur is oxidised. Then according to you sulphur is reduced also, which i don't think is happening
Ox state of sulphur is diff.
s2o3 is thio -sulphate ox. stae of S is +3 & +1
s4o6 is also thio -sulphate ox.state is +3,+2,+2,+3
for ques 2
I2+Na2S2O3→Na2S2O3+I-
This is the imp. reaction I was talking about
IS Q. 2 CORRECT ????????????
what's the comn reaction & reagant use ?????????
yes kartick you are right in ques 4
3) can be balanced by hit and trial method so better show the steps of balancing by oxidation number method
4) A & C :
as there is no e° in 4s orbital so ground state is not possible as 4s orbital has low energy so e° will filled firstly.
It can be catonic state as there is no e° in 4s (eg. Cr+ ,Mn2+)
Q7 2AgCl + Na2CO3 → Ag2CO3 + 2NaCl
initial millimole 7.5 0 0
final 7.5-a a 2a
[Cl-]=0.0026/35.5= 2a/5
a=1.8*10-4
now [Na2CO3]=7.5-1.8*10-4
=7.5
[Co32-]=7.5
Ksp =[Ag+]2[CO32-]
calculate [Ag+] and then calculate KSp
think more
hint sulphur is oxidised from S2O3-2 to S4O62- a well known reaction