as there is no unpaired electron so it will be DIAMAGNETIC.
Bond Oreder = No.of bonding electrons - No.of antibonding electrons /2
i.e.= 6-4/2 =1
So the ans will be (a)1 and diamagnetic
Assuming that Hund's Rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
a.1 and diamagnetic
b.0 and diamagnetic
c.1 and paramagnetic
d.0 and paramagnetic
-
UP 0 DOWN 0 0 2

2 Answers
Apply MOT here.
Step 1 - Write down the actual distribution of electrons in the molecular orbitals for B2
Step 2 - You will find that the 10 electrons (5 each from each B atom) are distributed as: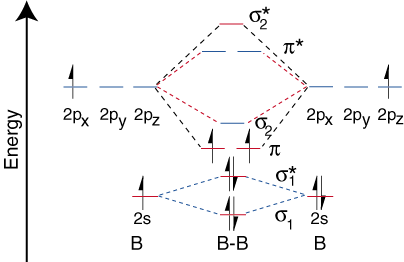
Step 3 - From here calculate the Bond Order (No. of electrons in Bonding - Antibonding Molecular Orbitals)/2 and magnetism (paramagnetic if there are unpaired electrons or else diamagnetic)
Step 4 - Think of how this distribution of electrons would change if Hund's Rule is violated.
I have told you the approach. Now you let us know your answer.