24
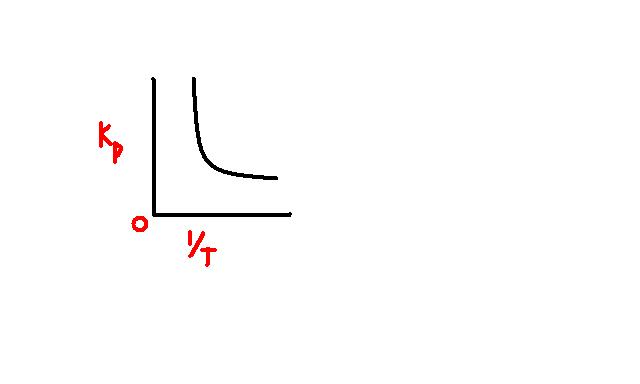
24Since it is rectangular hyperbola,
K_P.\frac {1}{T}=c \ where \ c \ is \ a \ constant
=> K_P=cT
Also \ K_P=\frac {p_Z}{p_X.p_Y}
where pi denotes respective partial pressure
Now as T increases =>KP increases
so it is obvoius that Z will ahve higher proption at higher temp
 4
4But what abt high pressures
Should we consider that P is directly proportional to T ?????
 24
24yeah,,,,
since no. of moles(total) and volume is constant
so Pressure is directly propotional to temp...and then same logic [1]
 Z
Z