in general any endothermic rxn can be
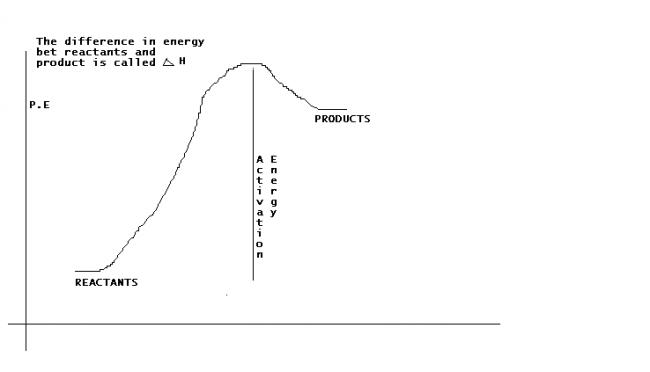
For an endothermic reaction where ΔH represents the enthalpy of the reaction,the minimum value of the energy of activation will be
a) <ΔH
b)>ΔH
c)=ΔH
d)0
this is a very nice question
i think c. .. the activation energy is jus sufficient to reach the product (see the nice graph above)...
(if b had to be the ans,,,, it wud not have been a question :P
this is not the reason y i told c but jus felt this.... )
no u are not wrong...
your graph is a correct one no doubt... but dats wat happens generally...
but the question asks fro the min act energy... so i wonder if something new happens...
This is an ex-IIT question.
The ans is (b)
We can say this bcoz in any case the activation energy has to be greater than the enthalpy as it can be clearly seen in Sankara's graph
here we have to choose the best option
no doubt the limiting value for min activation energy is ΔH but in case if (b) option is given we should choose (b) if not we will choose (c)