1) The last one shuld be rite.
(Always remember ki Rate Law exp. mein Intermediate specie ki conc. nahi aani chahiye)
1)Nitrogen monoxide, NO, reacts with hydrogen, H2, according to the following equation:
2 NO (g) + 2 H2 (g) → N2 (g) + 2 H2O (g)
If the mechanism for this reaction were, 2NO(g) + H2(g) N2(g) + H2O2(g) (fast) H2O2(g) + H2(g) → 2H2O (g) (slow)
which of the following rate laws would we expect to obtain experimentally?
Rate = k[H2O2][H2]
Rate = k[NO]2[H2]
Rate = k[NO] 2 [H2]2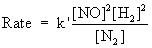
2)Order of a reaction is an experimental quantity. One of the ways of determining order of a reaction is plotting graphs of concentrations of reactants obtained at different intervals of time. A plot of log[Reactant] vs time (min) resulted in a straight line with a slope of – 0.0218.
The rate of the reaction when the concentration of the reactant is 0.3 M is
0.015 M.min-1
0.00654 M min-1
0.05 M.min-1
None of these
-
UP 0 DOWN 0 0 2

2 Answers
i know that but i am not able to derive the rate law expression so if you could plzz post the working it would be great ....