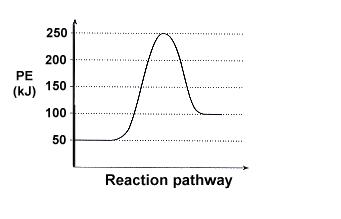
a) The reaction profile represents an endothermic pathway as from a lower state of energy, further energy is absorbed to get to a higher state of energy as the energy hill shows.
b) Reactants at 50 kJ, activated complex at 250 kJ, products at 100 kJ.
c) 50 kJ?
d) 200 kJ
e) 150 kJ