Yup !
Answers are correct !
The following curve is obtained when molar conductivity (â‹€m) is plotted against the square root of concentration for two electrolytes A and B.
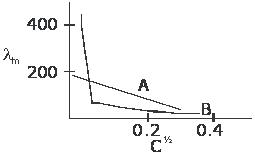
then ,correct answers are
(a) B is a strong electrolyte and A is a weak electrolyte.
(b) Molar conductivity of a strong electrolyte (A) increases with dilution as ionic mobility
increases
(c) A is a strong electrolyte and B is a weak electrolyte.
(d) B will dissociates faster than A.
-
UP 0 DOWN 0 1 2

2 Answers
Debotosh..
·2009-12-04 21:09:55
A is a strong electrolyte and B is a weak electrolye....and molar cond of A increases with dilution ....hence answers are (c),(b)
reason : no extrapolation for the graph of B is possible whereas we can extrapolate the graph for A to get Λm∞