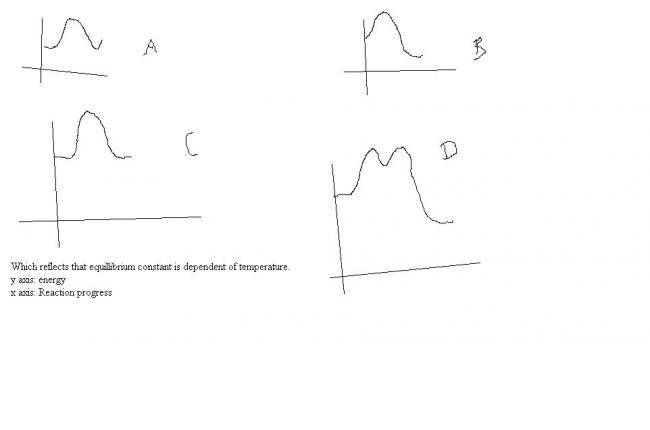
3
3SEE... EQ. CONSTANT IS BASICALLY Kf/Kb...................
by arrehineus eqauation K = A eRT/Ea....
Kb/Ka =eRT(1/Eaf-1/Eab)...........
firstly it depends on whether energy of activation in forward direction is greater than or equal to ot lesser than energy of activation in backward direction!!!!!!!
or in simple words whether the rxn is exothermic or endothermic!!!!!!!!!
correct me if im rong!!!!!!!!!!![1]
 3
3oh!!sorry i didnt see that it was an eneergy curve........ so based on my above explaination it shud be b...............because initial and final energies have to be different for T to play a role.......... see the eq. in mah prev. post!!!!!![1]
 1
1i also think b. ...not sure... at first glance...
 1
1from arrhenius eqn,
delH= - RT lnK
so del H <0 => process is exothermic.
and in the graphs given only B shows exo rection..
 3
3he says that K is DEPENDANT on temperatur so it cud be anything in which del H ≠0 .........
 1
1ofcrz it could be....
well if its multiple choice, both A and B shud be the ans... i think..
 3
3i wud say ABD .......... cuz even D depoicts a del H .......
 1
1D is somewat like SN1 reactions.. [3]
am confused wid dat..
A,B dono pakka hoga....
 3
3skygirl me thinks D rep. a sequential rxn. must have heard of it in chemical kinetics!!!!!![3]
 1
1sorry guys... answer is C..even i don't know how it came(even i thought of B)
 3
3ur question shuda been """independent""" of temperature .... then C is the answer........
check the question again......