Someone reply.... :(
Q:=> 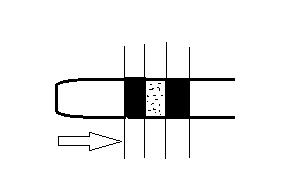
The tube contains 10 cm of air column and 4 cm of mercury column each with 4 cm of water column between them as shown above.
Now the tube is made vertical. Now find the the length of air column/water column?
Q:=> How do i find the rate of effusion of a reaction mixture after any time interval say t?
-
UP 0 DOWN 0 0 8

8 Answers
In horizontal position the system is in equilibrium which hints that there is no force in horizontal direction on the liquid column!
thus pressure in air column is Po i.e. atmospheric pressure.!
considering area of cross section A,
Po.A.10=nRT
when the thng is made vertical, to remain in equilibrium the net vertical force needs to be zero on the liquid column!
so new pressure P=Po + hHgÏHgg + hwatÏwatg
use P.A.x=PoA.10RT.RT
considering the entire thng to be an isothermal process! :)
so putting everythng we will get x! :)
tat is required!
now again let us concentrate on the lower mercury block!
the pressure on either side of this shud also be same!
same is the case for water column and then for the next mercury column!
For this we will consider the mercury thng to be non comprisible and non volatile!
but we should consider the vapour pressure of water while balancing pressure! :)
hope u can kill the question now!
But Pressureon either sides of lower mercury block ≠Pressureon either sides of upper mecury block.
So Water column must change na?
wahi to bola!
it will change!
and so u will have to consider the vapour pressure of water!
:-o
H C Verma
part 2
thermodynamics!
Kinetic Theory Of Gases!
Parh Le!
Kaam ayega! :)