sahi hai ya galat hai koi to bolo .
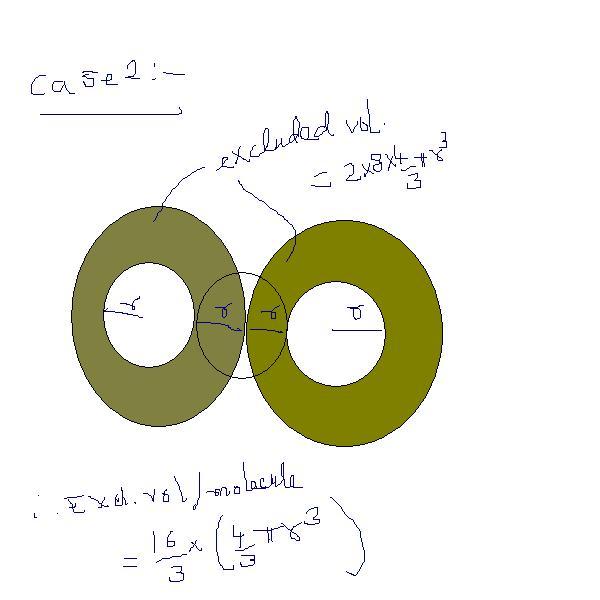
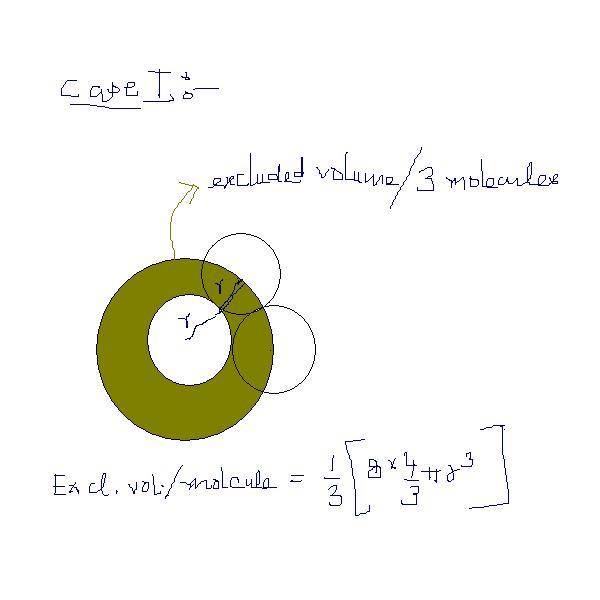
in case of bi-molecular collisions we calculate the value of b(in volume correction )as 8Ï€r3/3 (for one molecule) can somebody give me the value of b in case of tri-molecular collision and tetra-molecular collision
-
UP 0 DOWN 0 1 19

19 Answers
can any say how to solve problems in gaseous state easily??????????????
this thing u have got right and now i wonder why u r getting wrong ans the centers of these molecules will make a tetrahedral figure and sorry the ans i have posted is wrong i will post the right soln
it's 3D collision no ??
is it something like this??? mind you I've picturised only the collision.
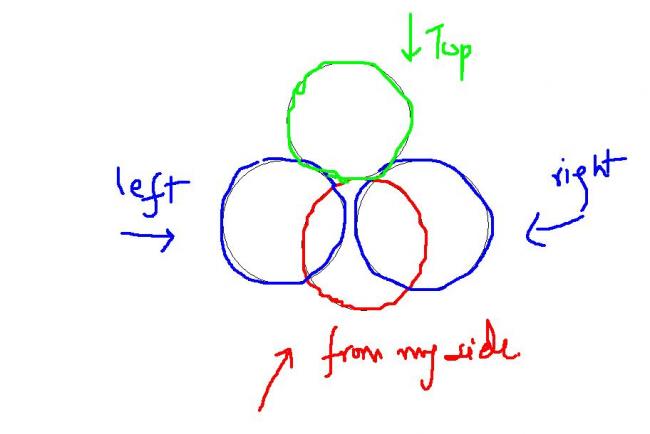
no . no ....
I have just shown why it's 4V for one molecule. that's all ....
the molecule can rotate about one point ..no . so it's now rotating about the center of bigger circle. now it's correct?
I'll try your Q now .
b is defined for one molecule only and i thin u r calculating b for tetramolecular collision ans is 31.5*2-3.5 V
where V is the volume for one molecule
this is for one molecule da . not answer for your Q .
for one molecule alos this is wrong ?
isn't the excluded volume equal to b=4V where V is V of one molecule.? coz of this I feel . for single molecule ?is this not it?
diag kharab hai . sry.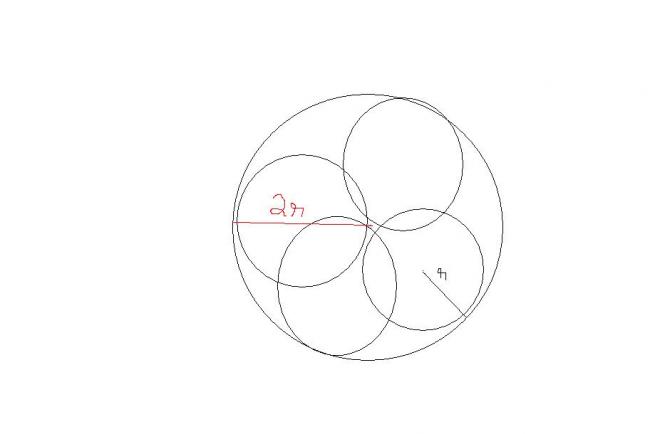
mam [7] [12] [12]
change.........
well about the reply.......
not mug up thing......
its lyk..... obviously the same idea as in bi-molecular...
i din thot of inventing sumthing new...
but now as u saying its diff...
i will try to think diff-ly...
mam the method u r applying is wrong basically u have mug up the soln for bi-molecular collision case so sorry but both the ans given is wrong