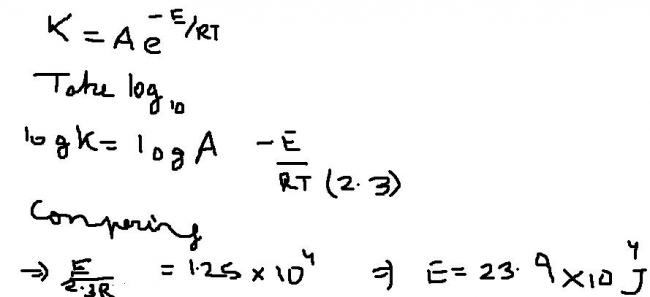rate = Ae-Ea/RT
lnk = lnA - Ea/RT
=> logk = logA - Ea/2.303RT
comparing the given eqn with the above one...
Ea/2.303R = 1.25E4
=> Ea= 2.303X8.314X1.25E4 J
half life = 256 mins = 256X60 s.
k = 0.693/[256X60]
wen we put this in the above eqn, we get T.