ANS 24√2 R*R*R
In hexagonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure, called hexagonal close-packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be ‘r’.
The volume of this HCP unit cell is_________________________.
-
UP 0 DOWN 0 0 10

10 Answers
3 spheres touch each other to form a triangle , Another sphere (of same radius) is kept on these spheres to form a pyramid structure.
try to find the height of the pyramid
This will help is solving your question
John let us analyse this one by one...
lets find the side of the hexagon..
it is 2r
now also see the height... the one way to look at it is that it will form a triangle with base
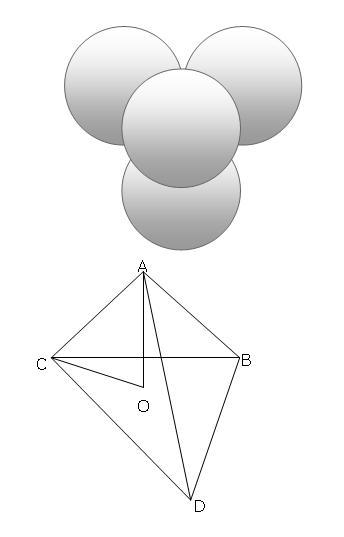
in this figure, BCD is an equilaterla traingle with side 2r
now the center O of the triangle is located.. find OB
now find OA..
you know the volume of one tetrahedral void!
Now what is the total number of tetra hedral voids in the whole HCP cell?
Now once you have found that simply multiply and find the answer :)
sir,plz solve it completely.i am unable to follow the guidelines given by u.