A=10*10*.....28 times
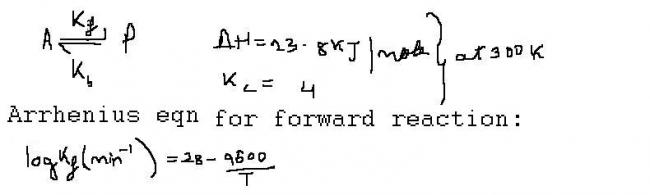
Q1 Calculate activation energy for formation of P ?
Q2 Calculate activation energy for formation of A?
Q3 What is arrhenius eqn for backward reaction?
-
UP 0 DOWN 0 0 6

6 Answers
i think i can xplain it we have kf=Ae^(-Ea/RT)
take log of both sides and compare with the given equation
activation energy for formation of P = 9600 x R kJ/mol,
activation energy for formation of A = dH + Ea(formation of P) (as itz endothermic reakn)
= 23.8 +9.6R kJ/mol
y here nothing pinked ?
i fully agree wid sids.
for the last part,
Kf = 10^ -4 (we get from the given eqn)
given , Kc = 4 => Kf/Kb = 4
form here we will find Kb.
and from previous parts we wud know Ea for backward reaction,
so we will put it there...
bus [1]
the term idependent T is log10A & i had not solve i had just seen tat u ask for A so solve it
K=A*e^-E/RT take log at base e then change to base 10 & compare to data u have given in question amd use R=8.314J/mole-K & if in kJ then R/1000