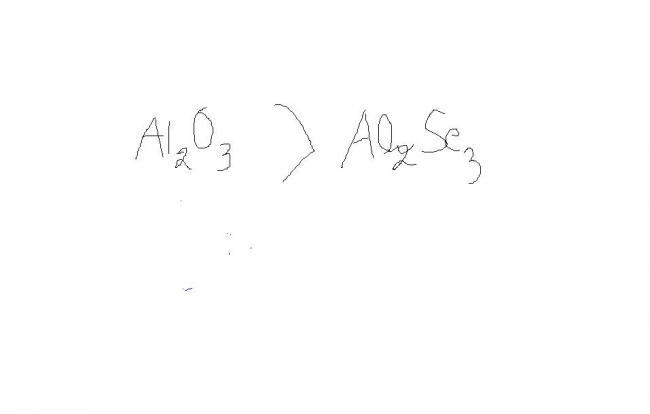1 Answers
Asish Mahapatra
·2009-08-08 02:10:13
Lattice energy depends directly upon the charges of the ions and inversely upon the sum of the distance between the cation and anion....
More ionic the compound, more is the lattice energy evolved
As the difference in electronegativity between Al and O is more than that for Al and Se so Al2O3 is more ionic hence its lattice energy is larger
Also, since the charges are same while size of Se>O so the lattice energy of Al2O3 is greater [because the distance will increase in case of Se]