bhai dekh
Fe-4s23d6
Fe3+-4s03d5
got it mate
we know that NH3 is a strong ligand.but then why complex[Cr(NH3)6]3+ have 3 unpaired electrons.
please give the complete and clear concept
-
UP 0 DOWN 0 0 41

41 Answers
I am leaving now
John Cena,rather than beating the hell out of Big Show study Coordination Compounds from NCERT
akshay coordination compounds naam ka chapter pada hai.u say agar 4 e- hote to pairing hoti.then wat about [Fe(CN)6]4-....haan.
oh,sorry i wrote wrong,wat about [Fe(CN)6]3-
what the hell happens here.here there are three unpaired electrons.why pairing takes place.
i think u need to study atomic structure also..............mate study again.
ya i was talking abt VBT (forgot the name [3])
but i think cena wants us to explain this q with respect to VBT
yeh mat poochna
+3 oxidation state kaise aayi nahi toh main sucide kar loonga
lols
dude your answer sucks...although u say there are 5 e- which is wrong but even here 5 is odd.
now correcting u in Fe 4s2 3d6 2e in 3d are already paired so it has 4 unpaired e-
when Fe3+ is formed config is 4s0 3d5 ...right but here 2e in 3d are already paired so there are three unpaired e- out of which 2 get paired and one is left unpaired.
akshay already suicided when his film chandni chowk to china was flop....................one of the worst jokes i ever made...hAAAA!!!!!!!!!
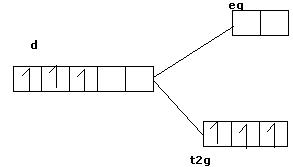
2 electrons already paired now 2 T2G orbitals left and 3 electrons,now apply kg 2 mathematics u will get the answer
lol
SUICIDE IS CORRECT NOT SUCIDE....
OH I UNDERSTOOD U WANTED TO SAVE UR LIFE IF I ASKED U FROM WHERE DID 3+ COME......THEN IF I ASKED U WUD DO SUCIDE NOT SUICIDE WHICH WUD SAVE U...............ANOTHER BAD JOD I EVER MADE.........HA!!!!!!!!!!!!!!!!!!!!!!
But here eg orbitals are of higher energy(octahedral complex) than T2g,so first T2g will be filled and follow Hund's Rule of max multiplicity so first all eg orbitals will be filled then pairing starts
hope u got it now machan
what cena says is:
NH3 is a strong ligand.....so it should cause pairing up of the electrons in Cr..........why is that not happening??
Do u know the concept of CFT,why pairing occurs it occurs because of energy diffrence of orbitals only
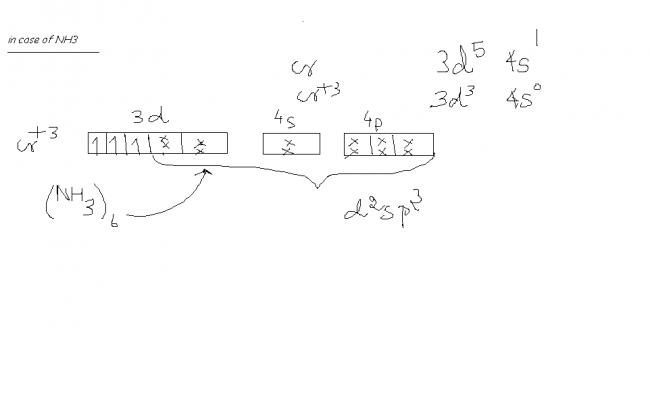
NH3 is a strong ligand ...hmm....but as far as i know it doesnt have dat capacity to pair up the unpaired electrons. as CN- has..so it takes new hybridized orbitals to accomodate its electrons..wait il show u.....
[9]
Akshay, u may also have heard that a strong ligand causes pairing up of electrons......which thus decides whether the hybridisation of te central metal atom...... sp3d2 or d2sp3
[11]
aarthi, any ligand above water in the spectrochemical series is considered as a strong ligand and results in the central metal atom having a hybridisation of sp3d2
strong field ligand bas splitting karta ha orbitals ki,pairing Aufbau Rules ke according hoti hai