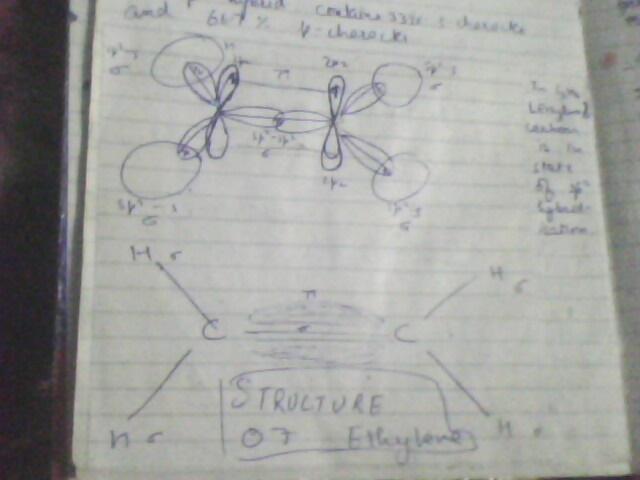
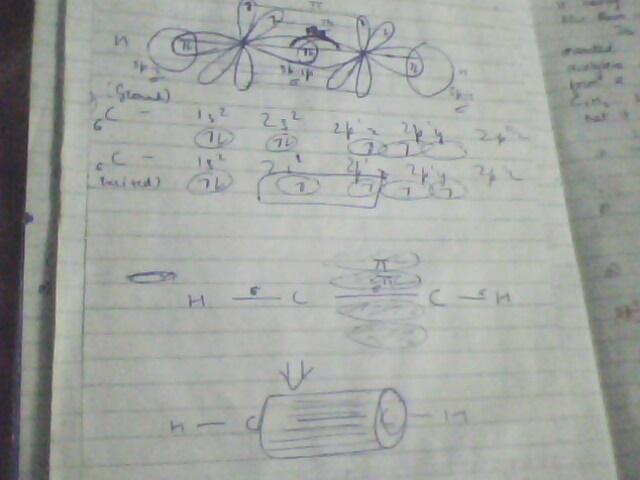
i hope the pics r self exlanatory...but if doubts still there, u may ask..best of lk
why rotation not possible in case of double bond but in case of triple bond ???????


i hope the pics r self exlanatory...but if doubts still there, u may ask..best of lk
there is a large energy barrier to rotation associated with grps joined by a double bond. Maximum overlap btw the p orbitals of a ∩ bond occurs when the axes of the p orbital are exactly parallel. Rotating one carbon of the double bond 90 degrees breaks the ∩ bond, for then the axes of the p orbitals are perpendicular and there is no net overlap btw them.
circular symmetry exists along the length of a triple bond. As a result there is no restriction of rotation for groups joined by a triple bod and if rotation would occur, no new compound would form...
hope this helps u...