explain this.
pls give the correct rate for the first order reaction.
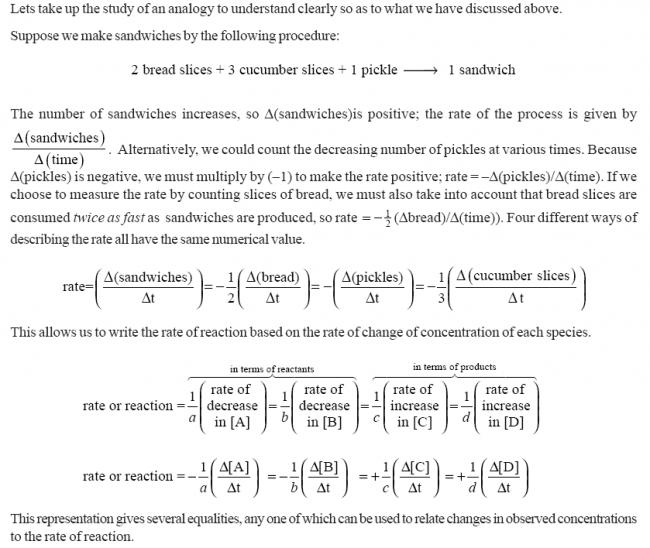
A→3B
is it d[A]/dt=d[B]/dt
or it is d[A]/dt=1/3d[B]/dt
-
UP 0 DOWN 0 0 7

7 Answers
Dr.House
·2009-06-01 10:04:14
its relative rates of disappearence of reactants or appearence of products
answer is 2nd option. mathematically , put a negative sign before dA/dt
Samarth Kashyap
·2009-06-01 19:04:34
rate of reaction is the rate of change of conc. of any if the reactants or products..
1 mole of A decomposes to give 3 moles of B in this reaction.
rate of change of conc. of A = rate of reaction.
but observe that the conc. of B increases 3 times more quickly than the conc. of A decreases. so rate of change of conc. of B is 3 times the rate of reaction.
so r=1/3 d[B]/dt