5. not sure . .. (1) ??
6. no idea
Q1 Change in vol delta V on mixing acetic acid (A) and benzene (B) is plotted against %benzene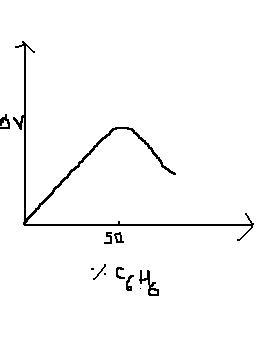
a)A-B bon dweaker than either A-A bond or B-B bond
b)A-A bond is weaker than B_B bond
c)B-B bond is weaker than A-A bond
d)A-B soln obeys raults law
Q2 vapor pressure o fsoln of non volatile solute B in solvent A is 95% of VP of solvent at same temperature.If mol wt of solvent is 0.3 times mol wt of solute,find ratio of wt of solvent to solute
Q3 T/F
1)all solutes are more soluble in H2O at high temp
2)solublity of solutes depend upon temperatuire
3)if liq solute is more volatile than solbent is added to solvent ,then the vapor pressure of soln >vapor pressure of pure solvent
Q4Which of the folowing correctly rep[resents thermodynamic properties during formation of 1mol of ideal binary soln
Q5 Which reperesents diff when non volatile solute is present in ideal soln
Q6 BP composoiton diag of liq vapor eq for A and B is shown.If binary liq mix of A and B is distilled fractionally,which of the following are correct ??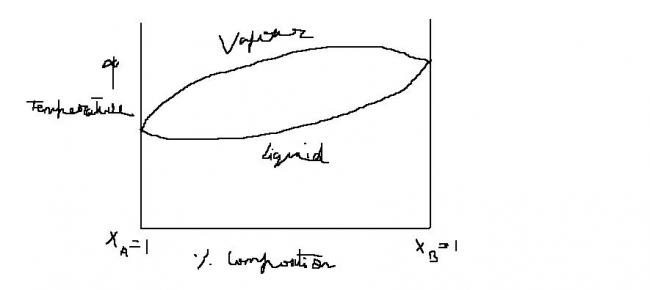
a)composition of still (residue )will apprach pure liq B
b)composition of distillate will paproach pure A
c)composition of distillate and residue will approach pure A and pure B respectively
d)neither of hte components can be obtained in pure state
Q1 a (delta V positive for solutions showing positive deviation)
Q2 0.95Po = XAPo
=> Xa = 0.95
=> ma/Mma/M + mb/0.3M = 0.95
in case of dilute solution,
0.3ma/mb = 0.95
=> ma/mb = 3.17
Q4. c .. for ideal solution delta H = 0, delta S (for all solutions) > 0 and delta G < 0
for question 2
p=p`x
and p=.95 * p`
so .95p`=p`*x
where x=mole fraction of solvent,p`=pure vapour presure of solvent p=v.p of solution with non volatile solut.
x=.95
moles of solvent/moles of solvent+ moles of solute = .95
moles of solvent(1-.95)=.95 moles of solute
wt of solvent/wt of solute=.95 * .3 / .05 = 5.7
ans=5.7
for ques 3.
1-false (because on incresing temp at certain temp. boiling starts it menas increase in temp increases the vapour pressure and if vapour pressure increases mole fraction of solvent increases so mole fraction of solute decreases which shows that solubility of solute decreases.)
above answer is only for non volatile solute..
in case of volatile solute it depends on volatility of solute
2-true
3-true