Line Defects
Line defects are mostly due to misalignment of ions or presnece of vacancies along a line. When lines of ions are missing in an otherwise perfect array of ions, an edge dislocation appeared. Edge dislocation is responsible for the ductility and malleability. In fact the hammering and stretching of maaterials often involve the movement of edge dislocation. Movements of dislocations give rise to their plastic behaviour. Line dislocations usually do not end inside the crystal, and they either form loops or end at the surface of a single crystal.
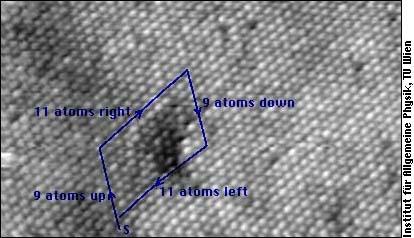
A dislocation is characterized by its Burgers vector: If you imagine going around the dislocation line, and exactly going back as many atoms in each direction as you have gone forward, you will not come back to the same atom where you have started. The Burgers vector points from start atom to the end atom of your journey (This "journey" is called Burgers circuit in dislocation theory).
In this electronmicroscope image of the surface of a crystal, you see point defects and a Burger journey around an edge dislocation. The dislocation line is in the crystal, and the image shows its ending at the surface. A Burger vector is approximately perpendicular to the dislocation line, and the missing line of atoms is somehwere within the block of the Buerger journey.
If the misalignment shifts a block of ions gradually downwards or upwards causing the formation of a screw like deformation, a screw dislocation is formed. The diagram here shows the idealized screw dislocation. This diagram is from the above link, which also shows an electromiscopic images of screw dislocations.
Line defects weakens the structure along a one-dimensional space, and the defects type and density affects the mechanical properties of the solids. Thus, formation and study of dislocations are particularly important for structural materials such as metals. This link gives some impressive images of dislocations. Chemical etching often reveal pits which are visible under small magnifications.
Example 1
The Table of X-ray Crystallographic Data of Minerals (The CRC Handbook of Chemistry and Physics) list the following for bunsenite (NiO): Crystal system: cubic, structure type: rock salt, a = 4.177*10-8 cm. In the table of Physical Constant of Inorganic Compounds, the density of bunsenite (NiO) is 6.67g / cm3. From these values, evaluate the cell volume (volume of the unit cell), sum of Ni and O radii (rNi + rO), 2 Molar volumes, (X-ray) density, and the Schottky defect vacancy rate.
Solution
Actually, most of the required values have been listed in the table, but their evaluations illustrate the methods. These values are evaluated below:
Cell volume = a3
= (4.177*10-8)3
= 72.88*10-24 cm3
rNi+rO = a / 2
= 2.088*10-8
X-ray density = 4*(58.69+16.00) / (6.023*1023*72.88*10-24)
= 6.806 g / cm3,
Compared to the observed density = 6.67 g / cm3.
The molar volumes (58.69+16.00) / density are thus
74.69 / 6.806 = 10.97 cm3; and 74.69 / 6.67 = 11.20 cm3
The vacancy rate = 6.806 - 6.67 / 6.806
= 0.02 (or 2%)