mere bhai! that was a memory test!!!
read POST #4
75.2 g of Phenol is dissolved in a solvent of Kf=14.
If the depression in freezing point is 7K then find the % of phenol that dimerises.
yes tapan.. you have to take mass of solvent as 1000g if not given!
YAAR TAPAN
AISE QUES MEIN STANDARD MAAN KE SOLVE KARNA CHAYIYE
AISE QUES CBSE NCERT MEIN BHI HAI
U CANT HELP
BUT REMEMBER TO TAKE MASS OF SOLVENT AS 1000g
75.2 g of Phenol is dissolved in a solvent of Kf=14.
If the depression in freezing point is 7K then find the % of phenol that dimerises.
Ther is no mention of MASS OF SOLUTE HERE ^^^
so the que. is a bit vague [2]
Sir, mass of solute kya hoga???
widout that v cant proceed ne.....
fir tereko ab doubt kiska hai
aggar 1000 g ka hai then i think nishant sir will be the best person to help us [1]
yaar standard maan ke chaala tha
i could be wrong
please rectify if u think my answer is wrong[1]
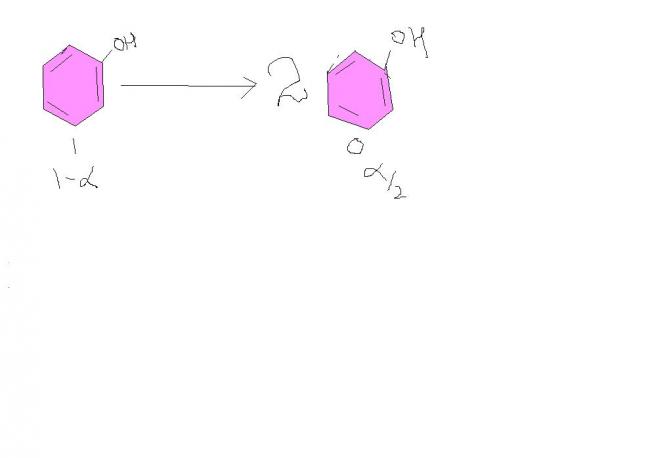
ΔTf=Kf X m
7=14X 75.2/94 X (1-α/2)
α=0.75
so 75 % phenol dimerises
sorry to fir mein bhool gaya final answer........
fir se karna padega........