sorry had missed out a sentence..[1]
In order to get maximum calorific output,a burner should have an optimum fuel to oxygen ratio which corresponds to 3 times as much oxygen as is required theoretically for complete combustion of the fuel.A burner which has been adjusted for methane.As fuel(with x lit/hour of CH4 and 6x lit/hour of O2)is to be readjusted for butane.In order to get the same calorific output,what should be the ratio of supply of butane and oxygen ? Assume the losses due to incomplete combustion,etc. are the same for both the fuels and the gases behave ideally.
Heats of combustion : CH4=809 kJ/mol ; C4H10 = 2878 kJ/mol.
(IIT 1993)
-
UP 0 DOWN 0 0 12

12 Answers
wat is the meaning when u say...."Assume the masses due to incomplete Heats of combustion : CH4=809 kJ/mol ; C4H10 = 2878 kJ/mol."???[1]
and wat is calorific value ??
is it energy per mole fuel or eneggy per gram fuel or energy per litre??
again i have another doubt..x lit of 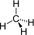 +6x lit of O2 is means molar ratio of 1:6 also....but to combust completely 1 mole
+6x lit of O2 is means molar ratio of 1:6 also....but to combust completely 1 mole  only 2 moles of O2...this thing hurts mee.plzzz explain..[1]
only 2 moles of O2...this thing hurts mee.plzzz explain..[1]
@subhomoy that is what is said that we have to take 3 times of the stoichiometric values to get max . output.
is the ans.
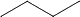 :O2 = 2:39
:O2 = 2:39
i
so we hav to jst balance eqn??
2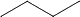 +13O2--->8CO2+10H2O
+13O2--->8CO2+10H2O
yes so 2 moles of 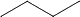 with 13X3 moles of O2
with 13X3 moles of O2
so it is 2:39...[1][1]
CH4+2O2→products
C4H10+(13/2)O2→products
Let the volume of 1 mole of gas be V.
energy released by x lit of methane=809x/V
energy released by y lit of butane=2878y/V
as energy released is same we have
809x/V=2878y/V
y=809x/2878
amount of oxygen required, z=(13/2)*y*3=(13/2)*(809/2878)*3