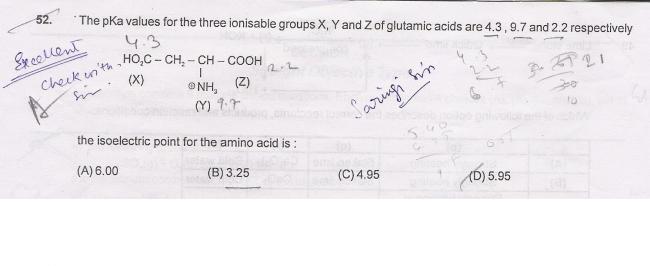but ionisation of Y decreases the charge. and of X Z increases the charge.
15 Answers
Iso Electric point is the pH at which a particular molecule or surface carries no net electrical charge.
Let the conc of H+ = x
Let the conc of OH- = 10-14/x
let the dissociation of x be α, of z be β. Then of Y will be α+β
10-4.3=[H+][α]/[1-2α-2β]
10-2.2=[H+][β]/[1-2α-2β]
10-2.2+10-4.3=[H+][α+β]/[1-2α-2β]
10-14/10-9.7=[α+β]/[1-2α-2β] 10-14/[H+]
[H+]/10-9.7=[α+β]/[1-2α-2β]
Eliminate
10-2.2+10-4.3=[H+][H+]/
10-9.7(10-2.2+10-4.3)=[H+][H+]
you get [H+]=5.95
See if this is the right answer...
for no net charge...
because if X dissociates by α.. then there is a -ve charge created
same with Z due to dissociation by beta
So for total net neutral charge the ionisation of Y should be α+beta
See if this logic is right
you guys anyway know how good my chemistry is :D
yes exactly..
so the net charge has to be zero.. is that only possible if amount of decrease is equal to amount of increase?
http://targetiit.com/iit_jee_forum/posts/try_try_4355.html
isme bhi same question hai.. aur answer sab dusra diya hai..
If amount of decrease is equal to amount of increase then finally net charge is same as initial charge, not zero.
I saw that post...
basically if there is a solution.. then you have equilibrium constants..
the total charge will not depend on where you start with!!
will it?
Even there I think you have to consider the equilibrium of NH3+ species at that pH.
I think sky has ignored that altogether.... !!
I saw that post soon after posting the solution to this one....
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch27/ch27-1-4.html
isko dekhiye naa... isse B aa raha hai..
in that question..
pKa2 is the equilibrium constant of some other reaction.. between two species...
But then I am not very sure.. you know how good I am at chemistry..
abhee Ihave to go.. when I come back i will think more on this one..
by that time, manish is trying this one :)
well the proof of this is given in the link that priyam posted.. but i did not understand it!!
you can read the proof and explanatin there :)
Yeah answer will be (pK1+pK2)/2
As the molecule required is HOOCCH2CH(NH3+)COO-
which is between HOOCCH2CH(NH3+)COOH and -OOCCH2CH(NH3+)COO-
so no Ka3 is required