1 = CH3F,
2 = CH3OH,
3 = HCOH,
4 = CH3COOH.......................due 2 deir b.p. (as v.p. depends inversly on b.p.)
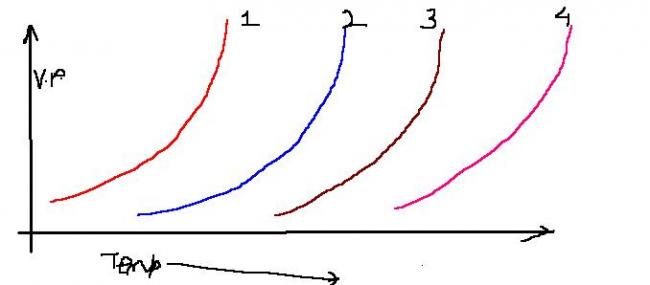
The following diagram shows vapour pressure curves for CH3F,CH3OH,CH3COOH,H2CO.
Match the curve with corresponding compound
-
UP 0 DOWN 0 0 16

16 Answers
it can be thot of in terms of volatility.
more volatility ... more vap pressure..
intermolecular H- bonding inversely proportional to volatility..
now here, H-bonding extension --- CH3F > CH3OH > CH3COOH > H2CO.
also, volatility increases with temp ... H-bonds break..
sooooo,,,, 4- CH3F
3- CH3OH
2-CH3COOH
1- HCHO
the one most volatile will have considerable vep pressure at lower temp.
note that, the vap pressure curve here is same for all,,,, jus some attains it at lower temp, other at higher temp n so on..
as b.p. depends upon (all dose factors , which skygirl mentioned).... voltality,intramolecular n intermolecular H - bonding + surface area, vander waal's force of attraction, mol. wt.......... so, i thought dat b.p. should b d best parameter which explain v.p. n temp. relation. n dats y i got dat order of v.p.............. ..................................but nw i think am rong, sorry 2 deviate u people. :( .... am apologizing :( but still i amn't able 2 gt any standard parameter to gt v.p. relation.......trahimam
can anybody tell me what a solution in pink stands for
and if for correct one why the soln of skygirl is in pink
[3] lol [9]
am i not correct....
then i am really sorry....
pls let me know the correct thing...
please..
methyl flouride is showing highest H bonding then only u can be right but let me know if that is true
y not methyl fluoride?
F has the highest e-negativity n smallest size.
dats y i thot it should be the strongest
my ans is
1→ CH3F
2→HCHO
3- CH3OH
2-CH3COOH
first two are gas at normal temp while other are liq
absolutely correct sir.................soory sky u were wrong............