X is the oxime, Y is the diketone.
Thought of posting a little quickie here since I have the rights...
Predict the products and explain the mechanism for the following reaction(s) -:
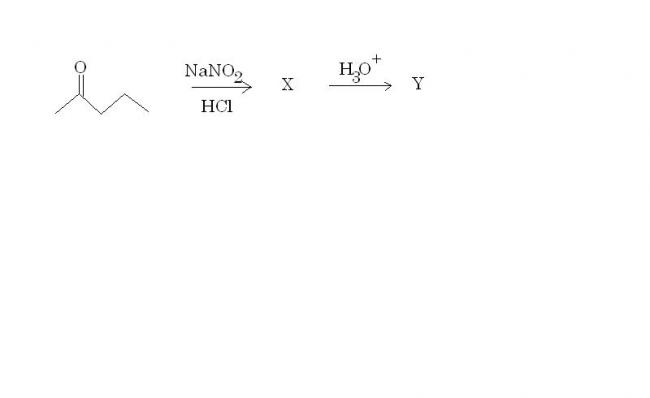
Note : Oldies to whom I have explained this reaction are not allowed to answer :P
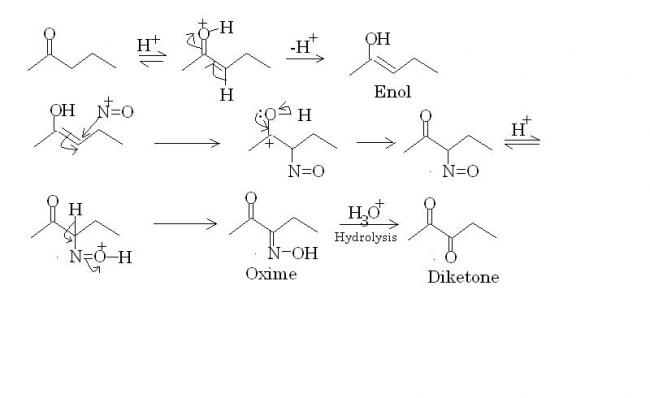
Hint : Think of acid catalysed enolisation as the first step.
No one upto it? Very well..I shall post the mechanism at the end of the day. Atleast try.
@uttara how did you know dat he pinked it himself :0
and abe is so not decent not here atleast
uttara...agar tumne answer post kari hoti toh us post ko main pink kar deta...agar tumhe jalan ho rahi hai toh main kuch nahi kar sakta [1]
pritish can u expain the mechanism of hydroslysis of an oxime
has it to do something with beckmann rearrangement
P.S i suck in chemistry , so may be the post is irrelevant
Sudharshan bhai...ankur asked me this question also. Hydrolysis of an oxime and Beckmann rearrangement are completely different. Hydrolysis can be done by different inorganic acids. Beckmann rearrangement is specifically done using sulfuric acid as a catalyst. Therein lies the difference. It's a good query though :)