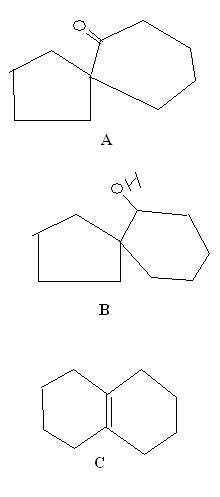
No one trying these? :O
Except ankur..
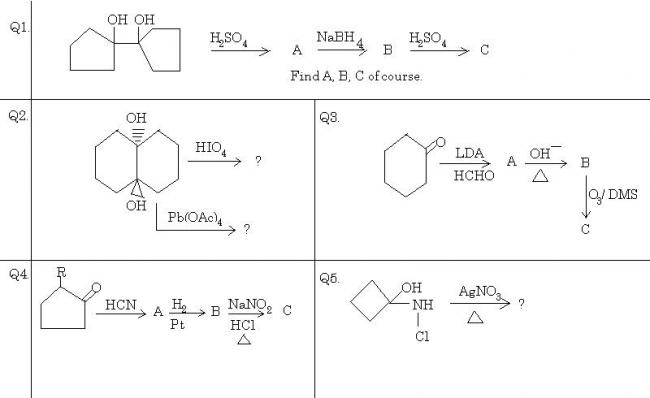
And finally, a bonus question -:
Q6. I have with me Br-(CH2)4-OH. I have to transform it into HO-(CH2)4-COOH. I can do this by the Grignard method(CO2) and by the Cyanide method(substitution and then hydrolysis). Which will work and which will not?
-
UP 0 DOWN 0 0 7

7 Answers
Answers -:
Q2. HIO4 has no effect on trans diols, and can oxidize only cis diols. Pb(OAc)4 oxidizes both types of diols, and hence the answer is a diketone in the second reaction, and no reaction occurs in the first.
Q3. It is a simple directed aldol condensation followed by ozonolysis. I won't list all the products here, but the final product is 2-oxo-cyclohexanone.
Q4. Addition of HCN across C=O, reduction of -CN to -CH2-NH2 and the rest is easily found out. The final product is a six membered ketone.
Q5. Five membered ring forms here. Silver causes the reaction to proceed forward. Find out yourself.
oh nice one pritish !
i remember now the transition state of oxidation of diols using HIO4 cannot go for trans like position
it will become highly strained