ok... but then it will be like
say,,, d=a - 2R = 4R/√3 - 2R = space left as voids..
now if d=2r, then r=2R/√3 - R
....
now am i right??
In a Lattice structure,
The atoms have small spaces between them. These spaces are filled by another atom! (of course smaller in size) so that they just touch the larger ones(These smaller ones are the largest ones possible without changing the position of the larger ones!)!
So what happens is that we have 2 atoms. The main atom and the secondary atom(filling the interstitial area!)
YOu need to find the packing fraction for
Simple cubic, body centered cubic, face centered cubic and hexagonal.
More over, find the second smallest distance between atoms in each such structure!!
PS: (Solve for as many parts as u can.. dont give just the answers.. some method... even the smallest is required)
I strongly believe... that if u can solve this one.. u can solve any question on this topic in JEE :)
-
UP 0 DOWN 0 2 25

25 Answers
wrong dude. this is not the solutin!!
where is the smaller atom!!!!!!!!!!!!!!!!!
In that case it will not fit in the crystal structure
because smaller sphere will push the body centred atom
the structure will become more distorted
but u c, y not fill up the way i did it before...
i mean taking the contact points at the corner spheres only??
.... then the packing fraction is more... so more efficient packing... isnt it?
plz explain...
Here you have done a little mistake
you have taken the small sphere in contact with the corner atoms
Now think how will it be in contact with the body centered atoms?
difficult???!!
weirdest question of the day....!!
mereko abhi tak nahi hua... kal itna try ki.. gav this question approx 3 hrs.. phir bhi nahi bana... :(
radius of smaller atom = (√2-1)R
is wrong Priyam :(
@integration.. nahi ye nahi nikalna tha :(
yaar this is thoda different.. read again !!
I thought this will be an easy.. but yaar this is turning out to be very difficult question....
hey bt yeh toh yaad rakhne wali baat hai na.
i mean for CCP and HCP itz 74%
for BCC itz 68%
and for simple cubic itz 52.4%
yehi nikalna hai na :?
Now tried for Body centered.........
(pahle chota waala try kar luu)
Ok radius of large atoms be R
side of cube = 4R/√3
radius of smaller atom = (√2-1)R
no of smaller atoms effectively in cube=3
packing eff=(4/3)pi*(2R3+3((√2-1)R)3)
----------------------
64R3/3√3
=.75
????????????????
Here we will use the formula a√3=2(r++r-) when the lattice atoms are at the corners and the central atom is at the body centre. Am I right Nishant. Can you see the hidden part if not tell me I will unhide it
Drawing its fig is too dificult...
I'll post it after sumtime...
if a is side length
and r is radius of sphere
then base area =6√3r2 now its ht is the main point...
OB=2r/√3..................(see fig which i'll post in a moment)
(what is angle BOA? this will make clear why OB=2r/√3.)
Now in triangle POB (P is center of face center)
PB=(OP2-OB2)1/2
OP=2r..........
PB=2r(2/3)1/2
ht of hexagon=2PB=4r(2/3)1/2
Vol of hexagon=base area * ht
=24r3√2
vol of atoms=6*(4/3)pi*r3
packing efficiency=vol of atoms/vol of hexagon
This is wrong..........:(
but i'll definitely try..........
hmmmm.................3d here i comes.....
sry abhisekh.. it was me who requested nishant bhaiya to jus solve for one part coz i was (still didn get ans though) unable to 'understand' the question :(...
yes...........
just returned home was outside frm morning.....
oh....... why u posted soln that early....
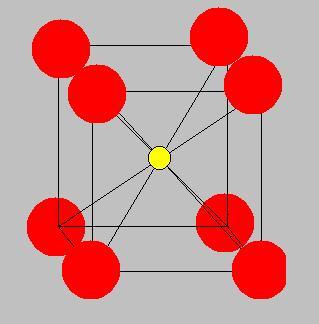
now we see for a simple cubic structure..
the void is at the center..
also, the void touches the 8 spheres.
Radius of red sphere is R
Radius of yellow sphere is r
so the diagonal = 2√3R
also, 2R+2r=diagonal = 2√3R
thus, r=(√3-1)R
inside one cubic lattice,
volume filled = 8×1/8×4/3pi.R3 + 4/3pi.r3
=4/3pi.(R3 + r3)
Volume of 1 lattice= (2R)3 = (2R)3
packing fraction is
4/3pi.(R3 + r3)/R3
= pi/6.(1 + r/R3)

i meant something like tis..
the red ball is in the air. .this is for hexagonal packing/cubic packing....
did u get my point?
Hey nishant is this what you want in the proof. I am sending this image just to show what I have understood. If you want something else please check and reply.
u are right essentially.
but i want the proofs. not formulas.. i dont think forumlas will take to too far in IIT JEE..
i mean good that u recognise that.. but i want the proofs for atleast a couple.. so that we are clear that u have understood the thing clearlly :)
and this formula will not suffice.. there is still more work to be done..

