i just want to know that what will happen if n is greater than 4 because if that so non planarity of the ring comes into play
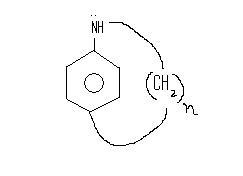
What will happen to the basisity of the compound with increase in n ?
-
UP 0 DOWN 0 0 25

25 Answers
but not much
the number of carbon atoms in the chain increases not that more alkyl groups are attached to nitrogen ..
one morething. I think the basibity of the compounds where n≥4 will be practically constant. as the stabilising factors are then very much the same for the compounds that follow. also to be noted is the fact that . N is occupying the Bridgehead position . thus due to inversion effect the compounds where n ≥ 3 are all significantly more basic than aniline .or NH3
Okay...it's time for me to answer the question.
First of all recall NH3 is more basic than 
That's because lone pair on N are defused into ring due to resonance.Now , the lone pair on N will resonate with the ring to attain more stable structure.After resonance N becomes sp2 hybridised.And the resulting structure will be triagonal plannar for N.
Ideal structure for N with sp2 hybridisation should look like this ,
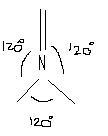
Now in the above figure we can see if n is large , the attainability of such a plannar structure of N will be easier , hence the lone pair of N would rather defuse to the ring to attain this structure as it can afford it.
On the other hand if n is small , let's say 1 or 2 , triagonal plannar structure would be very unstable as the bond angle can't be even close to 120 and hence the lone pair on N would be rather available , thereby increasing its basisity.
So with increase in n the N would rather attain plannar configuration defusing lone pairs to the ring and hence will be less basic.
Okay...it's time for me to answer the question.
First of all recall NH3 is more basic than 
That's because lone pair on N are defused into ring due to resonance.Now , the lone pair on N will resonate with the ring to attain more stable structure.After resonance N becomes sp2 hybridised.And the resulting structure will be triagonal plannar for N.
Ideal structure for N with sp2 hybridisation should look like this ,

Now in the above figure we can see if n is large , the attainability of such a plannar structure of N will be easier , hence the lone pair of N would rather defuse to the ring to attain this structure as it can afford it.
On the other hand if n is small , let's say 1 or 2 , triagonal plannar structure would be very unstable as the bond angle can't be even close to 120 and hence the lone pair on N would be rather available , thereby increasing its basisity.
So with increase in n the N would rather attain plannar configuration defusing lone pairs to the ring and hence will be less basic.
then are basicity of acid and this different ? If basicity of an acid is high, it can donate more protons and hence more acidic right ?
i dont think so
basicity = tendency of compound to act as proton acceptor(bronsted lowry concept)
wait .. basicity will decrease right ? If basic character increases, basicity will decrease right ?
first of all we need to consider the stability of the resonating structures
more stable the resonating structures are (with (+) on nitrogen),
more will be the positive charge on nitrogen(in the resonance hybrid as that is the ACTUAL structure)
and less basic it will be
now let us draw all the posiible resonating structures with (+) on nitrogen
there are two factors
i)due to positive charge on N more "n" will make the positive charge more stable
ii)due to more inductive effect of (CH2)n the para position wil be delocalised
as well the ortho positions by hyperconjugation
hence we can conclude that if it resonates , there will be an activating factor
and 3 other deactivating factors
if those three are more effective ..
then the compound's basicity will increase
if not
it will decrease ...
hmm.. now it is also attached to the 4th Carbon. when n increases, it increases the electron density on this C too... hence the para position will not be so stable ... hence it will deactivate ..
?
but rohan if there will be +I effect of (CH2)n on N there will be increase in the e- density in the ring due to +I effect...
one thing we can say
as "n" increases the resonationg forms of the compound which always contain a positive charge on nitrogen gain stability(+ve charge neutralised by them)
hence as "n" increases the lone pairs get more delocalised and thus less available in nitrogen
and thus less basic ...
it will increase bcoz....
(CH2)n will have some inductive effect....
so it inceases the e- density in the ring as well as near the NH...
now, since the e- density is inceased in the ring therefore the resonance effect of lone pair of NH will be highly reduced....
and also due to increased e- density around NH due +I effect of (CH2)n will force the lone pair of N to act as more basic and increase the basicity.....
hey satya is these lines the bonds....
or some direction of its attack....
With increase in alkyl groups, the electron density on N will increase, thereby increasing the basisity..