is it a PV curve?
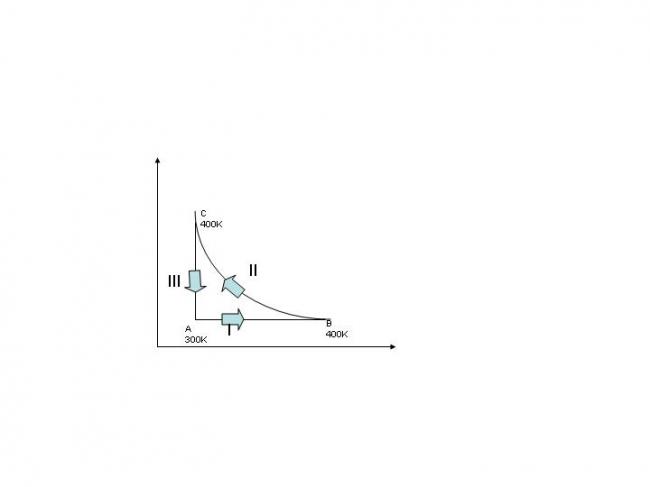
a mass of 8g of oxygen at the pressure one atmosphere and at temp. 27°C is enclosed in a cylinder fitted with a frictionless piston.the following operations are performsd:
I-the gas is heated at constant pressure to 127°C.
II-then it is compressed isothermally to its initial volume
III-it is cooled to its initial temp. at constant volume
Cv=670
1) what is the heat absorbed by the gas during operationI
2) what is the work done in stage A to B
3)what is the heat extracted fron the gas in stage C to A
PLZZZ EXPLAIN
-
UP 0 DOWN 0 0 2
