plz explain
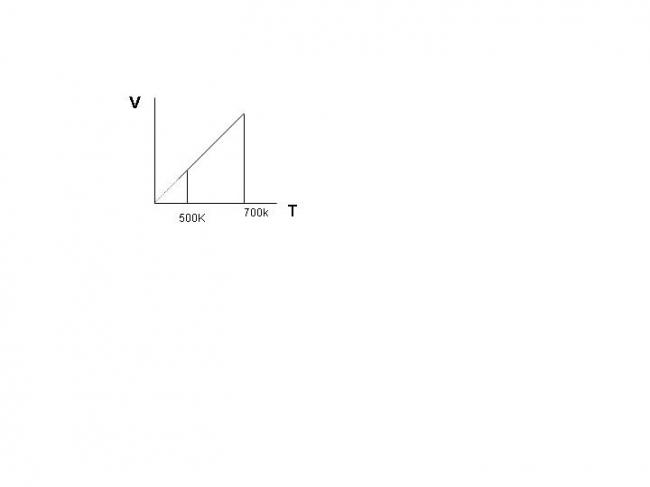
two moles of an ideal diatomic gas is heated in the process shown in figure
what is the heat given to the gas??
what is the work done by the gas??
-
UP 0 DOWN 0 0 1

1 Answers
Manish Shankar
·2009-04-10 21:15:00
The process is V=KT
V=(nR/P)T
so K=nR/P
here P is constant so isobaric process
heat given is nCpΔT where n=2, γ=1.4 and Cp=λR/(λ-1)
work done is PΔV=nRΔT