mercury is present in upper half completely??or onli a particular lenght??
A straight glass tube has sealed lower end and upper end open to atmosphere.The lower half is filled with n moles of diatomic gas .The length of the tube is 1.52 m
How much heat should be provided to the gas such that all the mercury is pushed out of the tube,given that
(Atm Pressure in SI units) * (Are of cross-section of tube ) * 0.76m = 16J
The answer given is 27 joules,how do you solve this?i was gettin 24J from a linear P-V plot
-
UP 0 DOWN 0 3 10

10 Answers
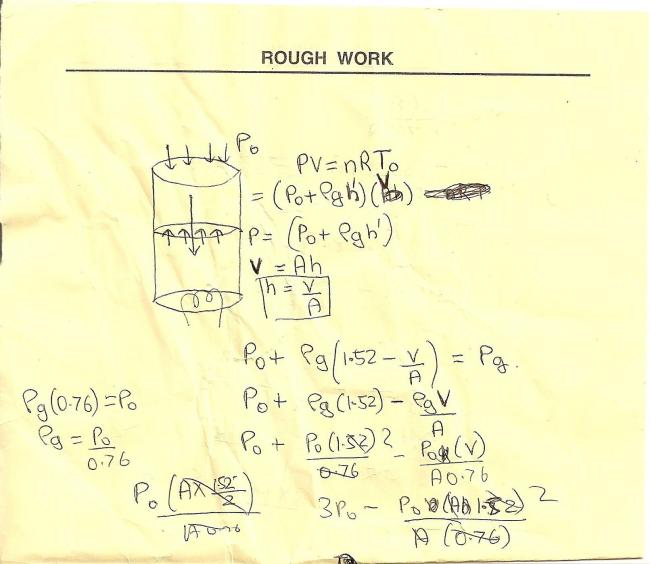

this is how i solved on paper too lazy to solve again hope u understand!!!!
im in 11 and this came in mah open test!!!!!!!!
 Anonymous Isn't h = 1.52 in the last stepUpvote·0·2020-03-01 13:11:49
Anonymous Isn't h = 1.52 in the last stepUpvote·0·2020-03-01 13:11:49
sorry itz all mixed up becuz i just rote it to solve it not to xplain to anyone!!!!!!
anyway wat ive done is that ive found pressure for a given vol. v and done PdV !!!!!!!!!!!!
i got 24 too,i did something similar,but the answer given by fiitjee is "27",or maybe its just a standard fiitjee goofup
no yaar it came in open test and even in the soln. key it was given as 24J so im damn sure!!!!!!!!!