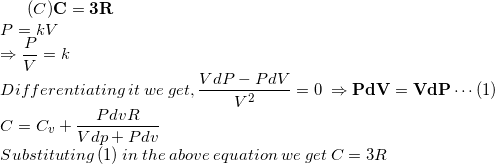
 Akash Anand Good job :)Upvote·0· Reply ·2014-04-24 00:16:11
Akash Anand Good job :)Upvote·0· Reply ·2014-04-24 00:16:11 Pratyasha Das THANK YOU ..BT CAN U EXPLAIN HW DID U GET "C=Cv + PdVR/Vdp+ Pdv
Pratyasha Das THANK YOU ..BT CAN U EXPLAIN HW DID U GET "C=Cv + PdVR/Vdp+ Pdv Aditya Agarwal dU=dQ-dW
nCv.dt=nC.dt-PdV
nC.dt=nCv.dt + PdV
C=Cv + (PdV)/ndT
PV=nRT => PdV+VdP=nRdT
C=Cv + (PdV.R)/(PdV+VdP)
Aditya Agarwal dU=dQ-dW
nCv.dt=nC.dt-PdV
nC.dt=nCv.dt + PdV
C=Cv + (PdV)/ndT
PV=nRT => PdV+VdP=nRdT
C=Cv + (PdV.R)/(PdV+VdP) Pratyasha Das ok gt it ..thanks again
Pratyasha Das ok gt it ..thanks again
 Anonymous caould you tell the rest of pdv=vdp....
Anonymous caould you tell the rest of pdv=vdp....