 1
1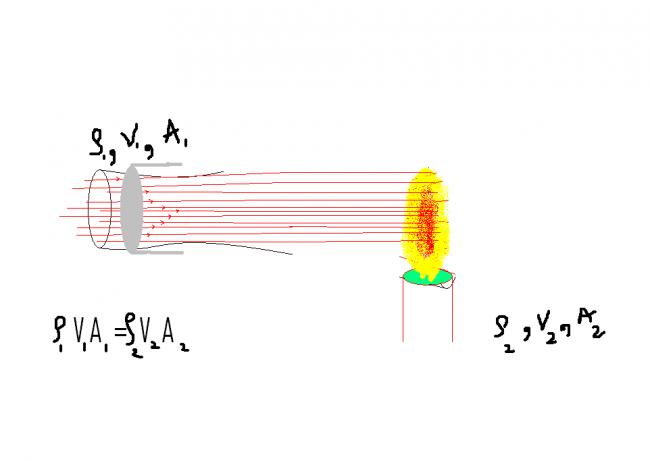
From equation of continuity ...
Ï1 V1 A1 = Ï2 V2 A2
* WHEN WE DO NOT BLOW AIR , THERE IS A STATE OF EQUILIBRIUM B/W AIR CONSUMED AND THE AIR THAT FLOWS IN... TO FILL THE VACUM AROUND THE ZONE OF FLAME,
* BT wen air is blown , considering a tube of flow , it is easy to observe..that ,
if we consider equal areas..
Ï1 > Ï2
, in order to compensate , air from surrounding no longer comes to fill.. in the vacum..so gradually ..air around flame bcomes less dense
 1
1THIS concept has only flaws..it is completely incorrect :( ...it is a shame that i did not analyse such a basic phenomenon :(
 11
11when we blow...the air temporarily creates a vacuum around the flame.the want of oxygen kills the flame..not the CO2.
 1
1For burning a candle, we require three things- Fuel (wax ), Oxygen and Ignition temperature.
But when we blow on the flame, it decreases the pressure around the wick (Bernouilli's Theorem) which in turn decreases its temperature of the wick. This causes the wick to solidify preventing it from reaching the combustion zone.
In short, blowing air (though it contains oxygen) causes interruption of fuel flow and cools the combustion zone below the ignition temperature.
 71
71In that way.. It should take many minutes to put off..
Shouldn't it depend on the atmospheric conditions too.?
LOL Imagine blowing a candle for 5 mins.
 1
1i do not know how can n e 1 attempt to solve this phenomn without taking into consideation the atmospheric conditions
