During Decarboxylation a negative charge appears on the alpha carbon ..... so all those compounds in which this negative charge is stabilised will undergo decarboxylation easily ....
So i think the answer will be a and b ... not sure about d .......
whats the right answer???
Which of the following compounds undergo easy decarboxylation?
(a)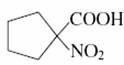
(b)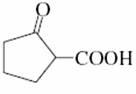
(c)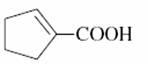
(d)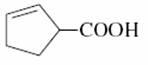
-
UP 0 DOWN 0 1 9

9 Answers
utd4ever
·2010-03-05 08:14:15
Pritish Chakraborty
·2010-03-05 08:30:43
I see utd...but in b), it's because of six-membered transition state.
Tapas Gandhi
·2010-03-05 10:26:43
A) acts as β-keto acid (N=O)
B) β-keto acid
D) β,γ - unsaturated acid
Tapas Gandhi
·2010-03-05 10:42:36
as utd says a -ve charge appears on α-carbon which in case of C) (here) or α,β unsaturated acid is not stable.
**** α,β → β,γ then possible
utd4ever
·2010-03-05 10:59:50
In c it is bcoz of the carbonylic carbon the negative charge on the carbon is accommodated on the oxygen which is more electronegative ....