2...EXPLAIN--
*BF3 HAS NO DIPOLE MOMENT BUT PF3 HAS SUBSTANTIAL DIPOLE MOMENT
*BF3 AND BrF3 MOLECULES HAVE DIFFERENT SHAPES
it is more ionic....if u c by fajans rule...since the anion is too small to be distorted by the strong positive charge of the cation.....
2...EXPLAIN--
*BF3 HAS NO DIPOLE MOMENT BUT PF3 HAS SUBSTANTIAL DIPOLE MOMENT
*BF3 AND BrF3 MOLECULES HAVE DIFFERENT SHAPES
2.BeF3 is sp2 hybridised n in same plane makin 120 degrees....so dipole moments calcel out...wheras PCl3 has pyramidal structure so the net dipole moment is not zero as in one hybridised orital there is lone pair
3.HOW ORTHOBORIC ACID IONISES IN WATER.....WHICH WAY OF WRITING FORMULA IS BETTER......H3BO3 OR B(OH)3
sumeet no offence but if u study NCERT u will get alll this answers....its given clearly in NCert
for 1.....BeF2 → BIGGEST ELECTRONEGATIVITY DIFFERENCE FOR A COMPOUND OF Be AND SO MOST LIKELY IS IONIC.........
BUT IT HAS VERY LOW CONDUCTIVITY IN FUSED STATE AND IS REGARDED AS COVALENT
3......H3BO3 IS SOLUBLE IN WATER AND BEHAVES AS WEAK ACID.IT DOES NOT DONATE PROTONS LIKE MOST ACIDS BUT RATHER IT ACCEPTS OH-..IT IS THEREFORE LEWIS ACID.AND IS BETTER WRITTEN AS B(OH)3.......it ionises in water giving B(OH)4- H+
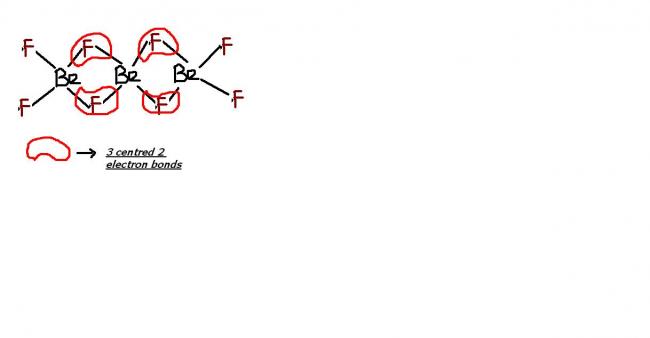
BeF2 is covalent !!! it exists as a polymeric structure with 3 centered 2 electron bridging bonds !
do u know about "polymeric" structures of group 2 compounds ?? BeF2 is one example of such clustering effect !!! we can extend this to BeH2 as well !