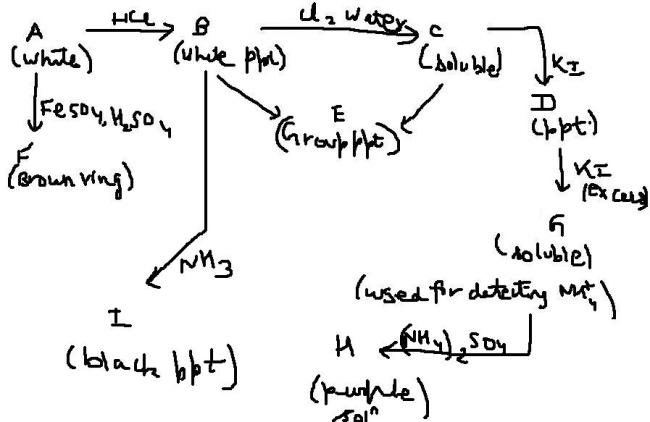
A is Hg2(NO3)2
7 Answers
A - Hg2(NO3)2
B - Hg2Cl2,
C - HgCl2 ,
D - HgI2 ( orange ppt. )
F - [ Fe (H2O)5 NO ] SO4 ,
G - [HgI4 ]2-
H - HgO·Hg(NH2)I ( IODIDE OF MILLON'S BASE )
NH4+ + 2[HgI4]2- + 4OH- → HgO·Hg(NH2)I + 7I- + 3H2O
I - Hg ( black ppt. )
Hg2Cl2 + NH3 → Hg ( black PPT.) + HgNH2Cl ( white ppt.)
E is perhaps HgS , as its referring to group precipitate, which is black in colour.
corrected. ya, u r rite. sorry fr making such a foolish mistake.
hope, u hv got rest of the answers.
"A" is giving Ring test => Anion - Nitrate...
Now fr the cationic part......
Since "B" is a chloride ppt. cation's one of Pb2+ , Ag+, Hg22+ (Only 3 main chlorides are insoluble in HCl i.e. the Group 1 Chlorides)
E (Dunno what they r actually asking for....)
In Ammonia (can even consider it as NH4OH)...Ag+ dissolves forming Tetra ammine silver cation, But Mercurous gives a black ppt of Hg with a white one of NH2.HgCl (but the overall effect is black!) ....so our cation probably is Hg22+ ion.....
The salt being Hg2(NO3)2.
Unable to view the rest of the img part...
OK.
A gives brown ring test. thus its surely nitrate or a nitrite. but nitrate salts are specifically used for standard reactions.
Now, the processes forming B, C, D , G, H clearly says dat G is Nessler's reagent. so, this clearly states dat the ion is either Hg+ or Hg2+
Now, Hg2+ on reaction with HCl gives HgCl2 , which is colourless. thus the cation is Hg+ giving Hg2Cl2 on reaction wid HCl which is a white precipitate.
then, HgCl2 is formed on reaction with Cl2 water, which is soluble. then, on reaction wid KI, HgI2 is formed which is an orange precipitate. and then Nessler's reagent is formed , which is used in the detection of NH4+ and gives iodide of millon's base on reaction wid (NH4)2SO4 in alkaline medium.
the two important reactions involved are :
NH4+ + 2[HgI4]2- + 4OH- → HgO·Hg(NH2)I + 7I- + 3H2O
and
Hg2Cl2 + NH3 → Hg ( black ppt.) + HgNH2Cl ( white ppt.)