@asish...sru it is enol form...as u said....din see.........
read enolate as enol......
so ans for 2 is abd
Q1. multi:
cooloidal gold can be prepared by
(a) bredig's arc method
(b) reduction of AuCl3
(c) hydrolysis
(d) peptization
Q2. multi:
Select correct statements
(a) Buring carbylamine rkn, intermediate is carbene
(b) During Dow's process, RI (rkn intermediate) is benzyne
(c) Enolate ion is RI in acid catalysed aldol
(d) Triplet carbene is paramagnetic
btw. is benzyne and carbene in syllabus?
Q3. single:
Debye Huckle Onsager equation is written as
\pi_{m} = \pi^{o}_{m} - (A+B\pi^{o}_{m})\sqrt{C}
A and B for water are 60.2 and 0.229, then in the folowing graph, HCl is ___
(the graphs are for AgNO3, NaNO3, HCl, KCl)
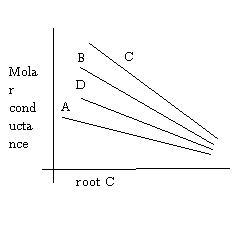
Q4. integer:
In a sample of two H atom, light of frequency which excite electron to 3rd excited state. Then max. no. of spectral line obtained in emission spectrum is x. x = ___
Q5. No. of pi-electrons in benzyne = ____
Q6. In H-atom, electron makes transition from n2 to n1 and can give 6 specrtal lines and max. no. of spectral lines obtained = 5. Then possible value of n1+n2 is ___ (integer)
Q7. How many types of stereoisomerism are possible for [Zn(CN)BrCl(H2O)]- _____ (does linkage isomerism count in stereoisomerism?)
@asish...sru it is enol form...as u said....din see.........
read enolate as enol......
so ans for 2 is abd
Ans 3..the reason i gave is that.. in comparison to all the cations of the compounds given H+ has the highest conductivity..the exact name of theory behind this phenomenon i din remember but the reason is that H+ in water displaces another Hydrogen from H2O and this phenomenon continues so H+ and OH- have very high conductivity....that's why the graph containing H+ as cation will have the highest conductivity hence C..
U may see the table below to compare the conductivity values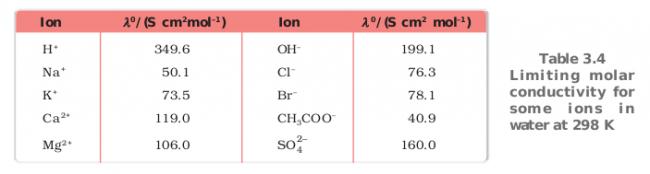
Source : NCERT
@govind: pls explain Q3. again
regarding benzyne : even our sir said 6 pi electrons (the triple bond... thing are not pi-electrons its just a partial overlap)
FINALLY:
Q1. ABD
Q2. ABD
Q3. C (to be explained by govind)
Q4. 5
Q5. 6
Q6. unsolved (ans given: 9)
Q7. 0
@manmay...maybe they wanted to ask...how many pi electrons participate in resonance in benzyne.........maybe a typo....
Achha one way fr one atom didn't click me...
But, rickde, agar hum 4→3→2→1 & 4→2→1 lein toh...waise 5 aayengi... & the Qn is asking fr the max. no. of lines...
How have we got the choice to decide the pathway..??
ans given as 2 for Q7...
HOW?? IT IS SP3 hybridised .. so no optical/geometrical
and unless u count linkage as stereo then u cant have 2
rickde aakash gives anwer that benzyne has 6 pi electrons.... ???
yeah that is what i had in mind too
i had also written 4 [1]
but Q6. is a bit confusing
4. it is 4...
the ground state is not an excited state....so 3rd excited state means 4th shell
for maximum lines...we send one H atom to ground state as
4→ 3 → 2 → 1
giving 3 different lines.....
second atom we send as
4 → 1....which is another line....so total 4 lines..
if we had one more H atom...we cud have sent it as
4→2→1 ..giving one more line...
@rickde: im pretty sure that acid catalysed is enol form and enolate is not formed..
the bruice that i have.. doesnt have acid catalysed aldol at all..
but u can see organic-chemistry.org
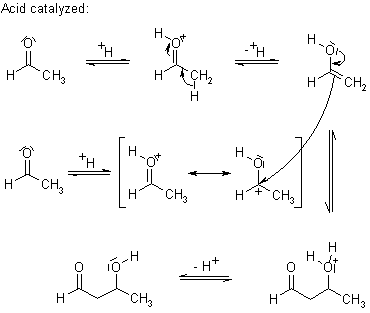
enolate is not formed
Aakash gives answer to Q1) as A ,B >...................Strange.
How can that be after looking into the NCERT and explanation it mentions is that it's a " fact "
1. A B D as govind told
2. a b d
EDITED
@Asish..in triplet carbene....the two electrons r not paired n r in different p orbitals.
posting answers given:
1. AB
2. all
3. C (govind pls explain again)
4. 4
5. 6
6. 9
wrong naa?
adding another question.
7. How many types of stereoisomerism are possible for [Zn(CN)BrCl(H2O)]- _____ (does linkage isomerism count in stereoisomerism?)
ans given 2
4) EDited : 3? (How wud the no. of H-atoms make ny sort of difference?)
5) Has 8 pi-electrons (though only 6 are delocalised).