GIVE THE NUMBER OF STRUCTURAL ISOMERS IN C3H4
in the electronic structure of sulfhuric acid , the total number of unshared electrons is .......
also explain it
-
UP 0 DOWN 0 0 7

7 Answers
jeetopper jee
·2010-10-07 08:53:46
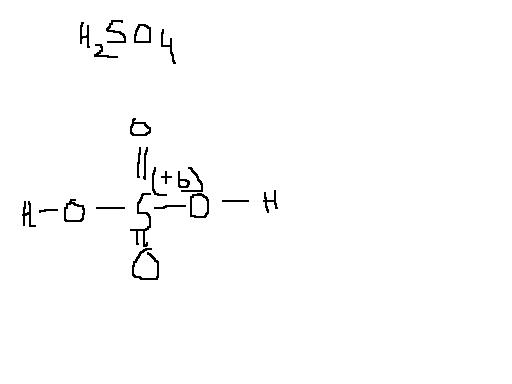 the structure of sulphuric acid is something as above.
the structure of sulphuric acid is something as above.
now very easily you can conclude that all the oxygen bonded have 4 lone electrons and thus 8*4=32 unshared electrons are peresent
Khyati
·2010-10-07 10:14:39
Well there are total 8 lone pairs(16 unshared electrons), 2 pairs( 4 unshared electrons) each
with one oxygen atom in Sulfuric acid.
Khyati
·2010-10-07 10:22:40
GIVE THE NUMBER OF STRUCTURAL ISOMERS IN C3H4
JEEtopper, I am able to make only two structure with this formula
1)HC≡C-CH3
2)H2C=C=CH2
Tell whether am right or wrong.
Pritish Chakraborty
·2010-10-08 08:17:35
There is one more, Khyati. Cyclopropene also has the same structural formula.