2..what happens when PH3 reacts with Ag+ ,Cu2+ ???
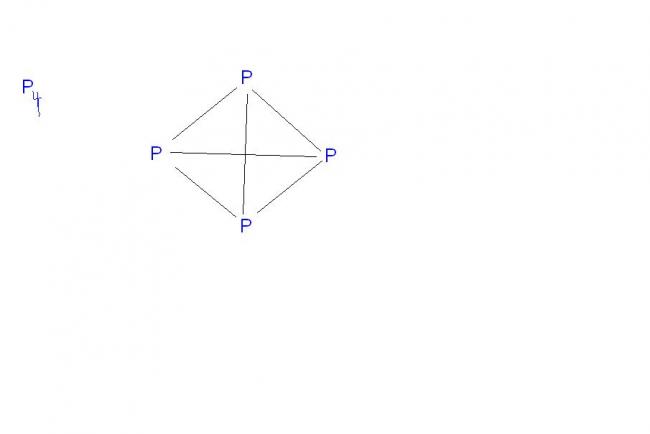
1.P4 exists but N4 does not...again N2 exists but P2 doesn't...why ??????
-
UP 0 DOWN 0 1 6

6 Answers
is the frst 1 related to presence and absence of d orbitals???give answer~~
1. nitrogen because of its small size & high electronegativity forms p∩-p∩ multiple bonds. Therefore, it exist as a diatomic molecule having a triple bond bw the 2 N-atoms. Phosphorus , on the other hand , due to its larger size & lower electronegativity usually does not form p∩-p∩ multiple bonds with itself.Instead it prefers to form p-p single bonds & hence it exists as tetrahedral P4 molecules
1.....i think this way
N2=946 KJ PER MOLE
N-N=160 KJ PER MOLE
P-P=215 KJ PER MOLE
P2=845 KJ PER MOLE
GREATER BINDING ENERGY GREATER STABILITY
COMPARE IT
In the second question metal phosphides are fomred...
Like Cu3P2 and Ag3P ...