To determine the structural isomers :
general formula :CnH2n+2O---------> represents isomerism between ethers and alcohols
general formula :CnH2nO----------->represents isomerism between ketones and aldehydes
general formula :CnH2nO2----------->represents isomerism between esters and carboxylic acids
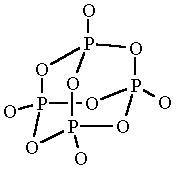
how to find the no. of sigma bonds in P4O10
and what will be it's structure?
what will be the no. of structural isomers of the formula C4H10O? how it is done?
what is the no. of isomers for the compound with molecular formula C2H3Cl3?
-
UP 0 DOWN 0 0 3

3 Answers
kartik sondhi
·2009-09-28 08:53:55
3)There are three isomers for this compound
2)There are 6 isomers two are in Ethers and 4 in Alcohols
Debotosh..
·2009-09-28 09:06:44