am i right ????
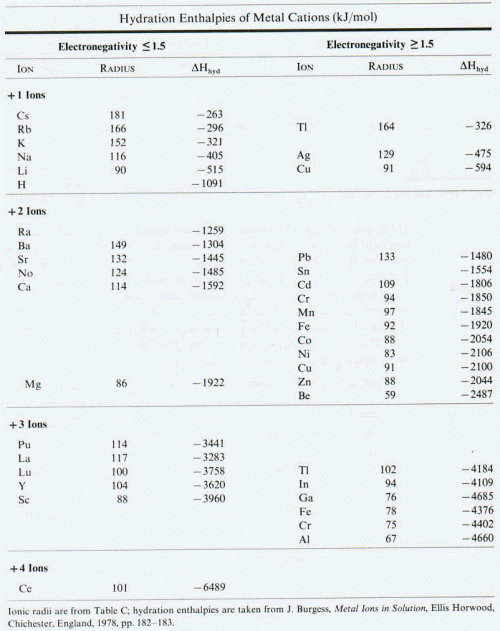
1) Among the following, the one having highest enthalpy of hydration is -
a)H+ b)Li+
c)Be 2+ d)Al 3+
2) For the balanced redox reac.n -
aNO3- + bAs2S3 +4H2O -------> xAsO4-3 + yNO + z3SO4 2- + 8H+
Identify the Incorrect statement (Single Option Correct) -
a) Equivalent weight of As2S3 =M/28 where M=its molecular weight.
b) a:b = 28:3
c) Value of (a+2b)/(x+y) is = 1.
d) All of these
-
UP 0 DOWN 0 0 11

11 Answers
ans 1> (b)
ans 2> (b) .......take the total change in oxidation number of the oxidized part !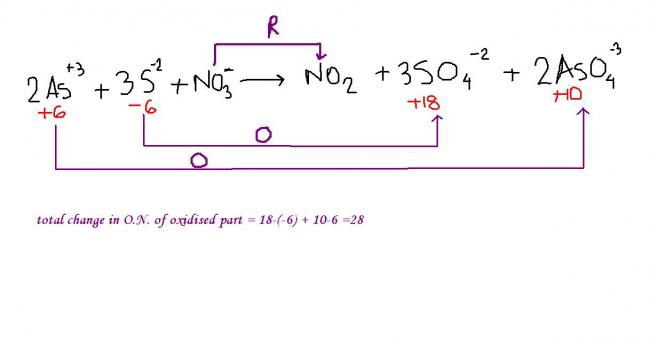
First one is correct .
for second ,
3 As2S3 + 4 H2O + 10 HNO3 + 18 NaNO3 → 9 Na2SO4 + 6 H3AsO4 + 28 NO
if we compare the above equation with your given equation then,
a =10
b=3
x=6
y=28
z=9
so,
now you can solve the problem .
I think this is not a single option correct problem.
check it , whether i am right !!!!!!
Yeah, i gave the same answers, but they went negative [138]
Anyways, the answers given were.......(d) for both...
Smaller the cation, greater is degree of hydration. Is that what you mean aieeee? isliye H+ ka sabse zyaada hydration enthalpy hona chaiye...
Lithium has highest hydration enthalpy within alkali metals. Maybe a proton should have greater h energy, as it is the smallest cation.
right. one more point :
Also , H+ forms coordinate bond with H2O , forming H3O+ ; which is a tronger bond than the hydrogen bonded Li+
well for (1) (d) is correct..
ethalpy of hydration is max. for Al3+... cant think of any reason though
found this and this
http://answers.yahoo.com/question/index;_ylt=AhVmJzBbJhaD4CKL1wcFEzzsy6IX;_ylv=3?qid=20091210010925AA0ieJS
http://www.science.uwaterloo.ca/~cchieh/cact/applychem/hydration.html
Googling helps a bit more here....
"The hydration enthalpy increases with increasing charge on the ion, whereas it decreases with increasing size of the ion."
Infact, Hhydration = - Ze2/ 2r*{1-1/d}
(Z=ionic charge, r=radius, d=dielectric const. of medium)
So v shud be able to use it like, whichever has a higher Z/r ratio shud have a more -ve value of Hyd. enthalpy.