Rajiv is right about question 3...that IS the dithionate ion..what makes you think otherwise, eure?
Achcha...now your question is to find the incorrect representations..lol. Ans a,c,d then.
Q1Boron readily dissolves in
a)conc HCl
b)fused NaOH at 673K
c)fused Na2CO3 +NaNO3 at 1173K
d)mixture of conc. HNO3 and conc. H2SO4 (1:2)
Q2 Nitrogen can combine at higher temp directly to give nitrides with
a)Mg
b)Al
c)Zn
d)Fe
Q3 Which is inocrrect representation of dithionate ion ?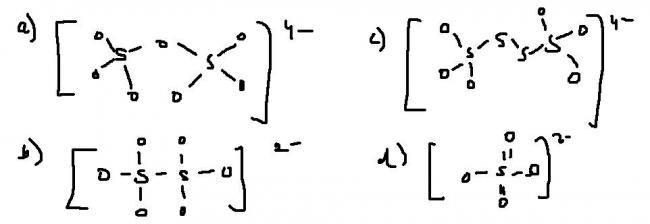
-
UP 0 DOWN 0 0 12

12 Answers
I think the answers should be:
1. a, b, c
2. a, b
3. Dithonate ion has a S-S bond...both S atoms are in the +5 oxidation state. Option (b) is correct
For Question 3 -I am absolutely sure that dithionate ion has the structure as given in option (b)
For Question 2, Mg3N2 & AlN are the two compounds that are formed. One needs highly electropositive metals for the formation of nitrides. Transition metals don't form nitrides in general.
For Q.1 - Let me check. Sure about Reactions 1 & 2 occurring... BCl3 & Borax are formed. Reaction 3 needs to be checked.
hehe ..ok ...looks like sir misunderstood teh ques and i misunderstood him...btw i still ahve dbt in strucure b)
dithionic acid looks like this naa ???
so how double bonds broken ...that too all ???
Q.3) Either its a printing mistake in the question or its trying to confuse us by giving the skeletal structures and representing every bond as a single bond.
Q.1) Answer is a , b and d.
a) B is not affected by conc. HCl in pure form at room temperatures, though, it shows some reactions with conc. HCl at very high temperatures.
2 B + 6HCl → 2BCl3 + 3H2
b) 2 B + 6 NaOH → 2 Na3(BO3) + 3H2
c) I hv not read abt such thing
d) Ya, this reaction takes place as the mixture of conc. HNO3 and conc. H2SO4 (1:2) acts as a very strong oxidising agent for B. this is also carried out by H2O2.
Q.2) The ans. is surely a and b.
Mg3N2 and AlN are formed. also, this reaction is shown by Li of group - 1 metals.

This is sodium dithionate..now you can judge for yourselves.
i can't say whether ' c ' is correct or not. but, a and b are certainly correct , as per J.D.Lee.
i hv also given the reactions involved .
ya i read that,,,.....maybe my book answer key may be wrong....but can u recheck once more plz ..