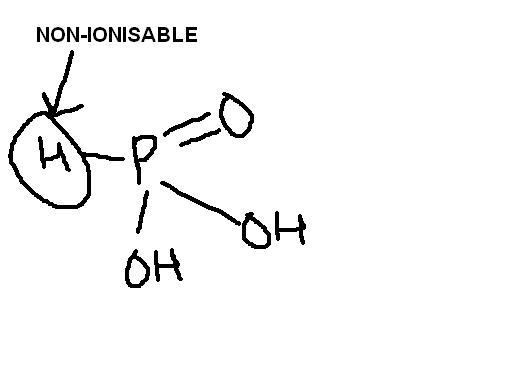
Non-ionisable P-H bonds toh reduction mein kaam aate hain...H cannot be separated as a proton. Instead it is given up as a hydride ion in a concerted mechanism of reduction. Those H's attached to oxygen are very much ionisable due to oxygen's electronegativity. There is one non-ionisable and two ionisable bonds here.
what is meant by non ionisable P-Hbonds. howmany non ionisable P-H bonds r there in H3PO3?
-
UP 0 DOWN 0 0 6

6 Answers
bcoz electronegetivity of p=1.8 and of H is 2.1
so, H is del -ve in P-H bond..if H is released, it will be released as H-(hydride ion)...ie h3po3 giving electron(as hydride ion) to other species..hence h3po3 is reductant...😑ðŸ‘ðŸ‘ðŸ‘ðŸ‘ðŸ‘
and It was given bond angle of PH3 is greater than that of NH3...i feel due to lonepair bondpair repulsions ....it is the otherway round...pls comment and confirm
i found something shiva in JD lee
that :-
H3PO3 contains 2 acidic H atoms ( the OH groups ), one reducing H ( the P - H Hydrogen atom ) . consequently only two of the three H atoms can ionize, and the acid is dibasic.
H3PO3 is moderately strong reducing agent.